Topic
Vitamin C
Contents
- Background
- Dietary sources of vitamin C
- Recommended intake of vitamin C for healthy people
- Vitamin C deficiency
- Shifting opinion regarding current RDA standard for vitamin C
- Vitamin C absorption and transport
- Vitamin C bioavailability
- Vitamin C for the use against the common cold
- Vitamin C and lung function
- Vitamin C and exercise
- Vitamin C and fatty acid oxidation
- Vitamin C and the brain
- Intravenous Vitamin C and its use for the treatment of infection
- Intravenous Vitamin C and cancer
- Effect of vitamin C on fertility and reproduction
- Intravenous vitamin C as a strategy to lower blood pressure
- Intravenous vitamin C as a treatment for heart attack-associated ischemia and reperfusion injury
- Vitamin C and inflammation
- Vitamin C's mechanisms of action
- Vitamin C safety
- Intravenous vitamin C safety
- Conclusion
Background
Vitamin C, also known as ascorbic acid, is an essential nutrient, widely recognized for its antioxidant properties. These properties arise from its potent redox potential due to its capacity to donate electrons to oxidized molecules. Even in small quantities vitamin C can protect critical molecules in the body such as proteins, lipids, carbohydrates, and nucleic acids (DNA and RNA) from damage by reactive oxygen species, which are generated during normal metabolism, by active immune cells, and through exposure to toxins and pollutants (e.g., certain chemotherapy drugs and cigarette smoke). The vitamin also plays a critical role as a cofactor – a molecule that assists enzymes in chemical reactions. This dual nature of vitamin C means that it is instrumental in multiple physiological processes, including those involved in the biosynthesis of collagen, carnitine, and catecholamines. As such, vitamin C participates in immune function, wound healing, fatty acid metabolism, neurotransmitter production, and blood vessel formation, as well as other key processes and pathways.
Vitamin C's role in immune function, in particular, is crucial. It stimulates the production of white blood cells, especially neutrophils, lymphocytes, and phagocytes, and promotes the cells' normal functions, such as their ability to detect, move toward, and engulf pathogens. Immune cells release large quantities of reactive oxygen species, often incurring damage. To protect themselves from this damage, immune cells accumulate large quantities of vitamin C, which serves as an antioxidant within the cells. Immune cells also release interferons, a class of proteins produced as a defensive response to viruses. Some evidence indicates that vitamin C promotes the production of interferon, a protein that participates in antiviral activity.[1]
Finally, vitamin C is involved in many other physiological processes. For example, it influences physiological levels of other vitamins. It regenerates vitamin E from its oxidized form and increases the bioavailability of iron from foods by enhancing gut absorption of nonheme iron. In addition, some evidence suggests that intravenous administration of vitamin C might be effective in treating certain types of viral infections and as adjunctive therapy in various types of cancers. Although many members of the animal kingdom can synthesize vitamin C, humans cannot and must obtain it in their diets.
Dietary sources of vitamin C
Vitamin C is widely abundant in fruits and vegetables. Although citrus fruits are synonymous with vitamin C, the highest vitamin C content (per 100-gram serving) is found in guavas, kiwis, and bell peppers. (Source: USDA) Vitamin C is easily destroyed by excessive heat and water, as well as exposure to air. Cooking foods destroys approximately 25 percent (or more) of the vitamin C present in foods.
Animal muscle meat is, for the most part, a very poor source of vitamin C. Organ meats such as cooked beef spleen, lung, and thymus contain moderate amounts (50 milligrams or less per serving) of vitamin C, but these cuts of meat are seldom eaten by the general public. A more commonly consumed cut such as corned beef brisket contains approximately 27 milligrams per serving when raw, but cooking will reduce that markedly, especially since corned beef must cook for several hours in a water bath. Of more than 950 beef items listed in the USDA's food and nutrition database, only 21 items contain any vitamin C at all, and most contain negligible amounts that would be lost in cooking.
Recommended intake of vitamin C for healthy people
The recommended dietary allowance, or RDA, for vitamin C varies according to age, sex, and life stage of healthy people. Needs are fairly high in infancy, decrease slightly during childhood, and peak in early adulthood. Pregnancy and breastfeeding increase a woman's vitamin C needs. Vitamin C is present in breastmilk, where it serves as an antioxidant for the mother's milk.[2]
The goal of meeting the RDA is to maintain an optimal blood concentration of approximately 50 micromoles per liter of blood to prevent oxidation of low-density lipoproteins.[3] The indicators used to estimate this requirement include the vitamin's capacity to provide antioxidant protection in neutrophils against reactive oxygen species produced during phagocytosis; prevent DNA and chromosomal damage; enhance immune function; facilitate collagen metabolism and carnitine biosynthesis; and maintain periodontal health.
A tolerable upper intake limit of 2,000 milligrams per day has been established to reduce the risk of gastrointestinal complications commonly associated with higher doses of vitamin C, such as diarrhea.[4] However, no scientific evidence demonstrates that vitamin C administered in doses up to 10 grams per day in adults is toxic or detrimental to health.
Recommended intake for special populations
People who smoke, consume alcohol, or have specific medical conditions commonly have higher vitamin C needs than healthy people.
Smoking increases oxidative stress, which increases antioxidant requirements.[5] Alcohol consumption increases urinary vitamin C losses by nearly 50 percent, suggesting that higher intake might be required to prevent deficiency in regular or heavy drinkers.[6]
"Alcohol consumption increases urinary vitamin C losses by nearly 50 percent, suggesting that higher intake might be required to prevent deficiency in regular or heavy drinkers." Click To Tweet
People who have inflammatory bowel diseases, such as Crohn's disease or ulcerative colitis, might have reduced plasma vitamin C levels, even if their disease status is quiescent.[7] In addition, people who have chronic renal failure commonly have lower plasma concentrations of vitamin C compared to healthy people, a condition that can be exacerbated by dialysis, which can trigger the production of reactive oxygen species.[8] A study involving 22 patients with renal failure and their matched healthy controls demonstrated that a single dialysis session decreased blood vitamin C levels even further. Prior to dialysis, average plasma vitamin C concentrations among the controls were approximately 42 micromoles per liter of blood versus 11 micromoles per liter of blood among the renal patients. After a dialysis session, however, the renal patients' average plasma vitamin C levels dropped markedly, to 6 micromoles per liter of blood, roughly half of their pre-dialysis levels.[9]
"After a dialysis session, however, the renal patients' average plasma vitamin C levels dropped markedly, to 6 micromoles per liter of blood, roughly half of their pre-dialysis levels." Click To Tweet
Similarly, a study in nearly 140 men (average age, 61 years) undergoing dialysis treatment found that low total vitamin C plasma levels predicted adverse cardiovascular outcomes. Patients whose vitamin C levels were lower than 32 micromoles per liter had approximately 300 percent higher risk of cardiovascular disease-related death. Patients with concentrations of 32 to 60 micromoles per liter had approximately 200 percent higher risk of cardiovascular-related deaths compared to patients whose vitamin C levels were greater than 60 micromoles per liter.[10]
Vitamin C deficiency
The classic manifestation of severe vitamin C deficiency is scurvy, characterized by bleeding, swollen gums, poor wound healing, joint pain, and bruising. Clinical features of scurvy appear in as little as 84 to 97 days of vitamin C depletion.[11] As scurvy progresses, a person might experience shortness of breath, dry eyes, joint swelling, weakness, fatigue, and depression. Obtaining less than 10 milligrams of vitamin C daily can cause scurvy. Interestingly, some disease states induce scurvy-level vitamin C status. The prevalence of deficiency varies across populations, with rates as low as 7 percent among people living in the United States, and more than 73 percent among people living in parts of Central Asia.[12] Interestingly, a population-based cross-sectional study of nearly 150 patients admitted to a large teaching hospital in Canada found that 60 percent of the patients had suboptimal plasma vitamin C levels and 19 percent were deficient, with levels approaching those associated with scurvy.[13]
Shifting opinion regarding current RDA standard for vitamin C
It is noteworthy that some scientists believe that compelling evidence supports increasing the RDA for vitamin C to 200 milligrams per day for adults. Whereas the goal of the current recommendations is to reduce the risk of scurvy, higher intake could saturate tissue levels, potentially reducing the risk of chronic conditions such as heart disease, stroke, cancer, and metabolic dysfunction.[14] A meta-analysis of 14 studies revealed that vitamin C intakes of approximately 450 milligrams per day markedly reduces the risk of all-cause mortality.[15] A meta-analysis of eight studies found that higher blood concentrations of vitamin C are linearly correlated with lower all-cause mortality risk.[15] Furthermore, research indicates that vitamin C intake of 1,000 milligrams per day nearly maximizes blood concentrations.[16]
Vitamin C absorption and transport
The absorption, distribution, metabolism, and excretion of vitamin C are complex and differ considerably from other low molecular weight compounds. Vitamin C is present in the body in either its reduced state (ascorbate) or its oxidized state (dehydroascorbic acid). Dehydroascorbic acid undergoes frequent intracellular recycling back to the reduced state.
Oral vitamin C is absorbed in the small intestine primarily via sodium-dependent vitamin C transporters and, to a lesser extent, glucose transporters. Absorption of the vitamin via sodium-dependent vitamin C transporters, or SVCTs, is dose-dependent and subject to transporter saturation. Dehydroascorbic acid competes with glucose for uptake via glucose transporters, or GLUTs, in the intestine and might be impeded in the setting of high blood glucose (as seen in diabetes).[17] However, most tissues do not use GLUTs to transport dehydroascorbic acid; rather, they use SVCTs to transport ascorbate. Red blood cells, which transport dehydroascorbic acid only and then generate ascorbate via recycling, are exceptions to this rule.
Upon absorption, vitamin C enters the plasma and then is widely distributed among the body's tissues. Tissue concentrations vary, however, with the lowest rates occurring in muscle, heart, and kidneys, and the highest rates in the brain and adrenal glands. The brain retains vitamin C during times of deficiency at the expense of other tissues, and animal evidence suggests maintaining vitamin C sufficiency is crucial during early brain development.[18] Vitamin C is metabolized in the liver to various intermediate molecules that can enter the pentose phosphate pathway (a cellular pathway involved in glucose metabolism) for further degradation. The vitamin accumulates in the kidneys and is excreted in the urine. Urinary excretion is directly proportional to plasma concentrations.

Vitamin C RDA.[4]
Vitamin C bioavailability
Oral vitamin C
The bioavailability of oral vitamin C is frequency- and dose-dependent. A person whose oral intake of vitamin C is approximately 200 milligrams per day from food or supplements will have plasma concentrations averaging around 70 micromoles per liter of blood. Evidence suggests that bioavailability increases at doses that exceed the current published tolerable upper intake level, however. Repletion studies have demonstrated that maximum bioavailability of vitamin C occurred at 200 milligrams when given as a single dose, but at 500 milligrams, bioavailability decreased, and excess vitamin C was excreted in the urine.[16] Even though bioavailability diminished after 500 milligrams per day, plasma levels still increased after higher oral doses up to 3 grams. Supplementing 1 to 3 grams of vitamin C once per day produced transient peak plasma concentrations that were two- to three-fold higher than those from 200 milligrams of oral vitamin C. These plasma levels saturated at approximately 220 micromoles per liter and returned back to baseline levels after 24 hours. Importantly, multiple high dose vitamin C supplements in the 2- to 3-gram range given four to six times per day maintained plasma levels two to three times higher throughout a 24-hour period.[19]
"Importantly, multiple high dose vitamin C supplements in the 2- to 3-gram range given four to six times per day maintained plasma levels two to three times higher throughout a 24-hour period." Click To Tweet
Liposomal vitamin C
A few studies suggest that oral bioavailability of vitamin C can be increased when consumed in liposomal form, which exerts a unique profile of oral bioavailability. A liposome is a lipid particle that can encapsulate substances such as vitamin C. Authors of a small, single-blind study involving two people provided different doses of liposomal vitamin C to the participants and then measured the participants' plasma vitamin C levels. Whereas a 20-gram dose increased peak plasma levels to approximately 320 micromoles per liter of blood, a 36-gram dose increased levels to 400 micromoles per liter.[20] A larger study involved 20 men and women between the ages of 31 and 65 who were given a single 10-gram dose of vitamin C, either in the free or liposomal form. The average peak plasma concentration of vitamin C in the participants who took the free form was approximately 180 micromoles per liter of blood. However, among those who took the liposomal form, the average peak plasma concentration was 300 micromoles per liter of blood, a 70 percent difference. Furthermore, the liposomal form took approximately one hour longer to reach the maximum vitamin C blood concentration, and its half-life was two hours more, compared to free vitamin C, indicating increased bioavailability.[21]
Another study in 11 men and women between the ages of 45 and 70 years compared the bioavailability of a placebo, intravenous vitamin C, oral liposomal vitamin C, and oral free (non-liposomal) vitamin C administration on four separate occasions. Peak plasma vitamin C levels depended on the method of administration[22]: Intravenous vitamin C produced average concentrations of 25 milligrams per deciliter of blood. Liposomal vitamin C produced average concentrations of 3 milligrams per deciliter of blood. Free vitamin C produced average concentrations of 2 milligrams per deciliter of blood. At least in terms of peak plasma concentration, intravenous was clearly superior. However, liposomal was superior in the context of oral administration.

Predicted plasma vitamin C concentrations in healthy persons after oral (top) or intravenous (IV) (bottom) administration of vitamin C.[19]
Intravenous vitamin C
Intravenous vitamin C bypasses intestinal absorption and the associated saturable transport mechanisms. Consequently, the bioavailability of vitamin C differs appreciably between oral and intravenous administration. In healthy adults, intravenous administration of vitamin C might reach blood concentrations that are 30 to 70 times higher than the same oral dose.[19]
A clinical study in which 12 adults between the ages of 19 and 27 were administered 1.25 grams of vitamin C either orally or intravenously, peak plasma concentrations reached 135 micromoles per liter of blood with oral administration but it reached 885 micromoles per liter of blood with intravenous administration. Furthermore, a high dose of 3 grams taken every four hours produced peak blood concentrations of 220 micromoles per liter of blood compared to 1760 micromoles per liter of blood for a single 3-gram intravenous dose.[19]
Vitamin C for the use against the common cold
The common cold, a viral infection of the upper respiratory tract that typically affects the nose, throat, and sinuses, is probably the most-studied application for vitamin C, due to the vitamin's role in maintaining proper immune function. Many clinical studies have investigated the effects of vitamin C in preventing and treating symptoms of the common cold, with substantial variation in the magnitude of benefit. Factors such as age, dose, and duration of supplementation affect the degree to which vitamin C can prevent or decrease episodes of the common cold.
A meta-analysis of more than 6,000 participants across 23 clinical studies explored some of the factors that could explain the variation in the magnitude of benefit for taking vitamin C during a cold episode. The criteria used to evaluate efficacy in each trial included the mean duration of symptoms and mean absence from work or school, if applicable. The analysis found that supplementation with at least 2 grams per day of vitamin C during a cold had a greater benefit compared to a dose of 1 gram per day. A sub-analysis of five studies found that higher-dose vitamin C supplementation elicited a more robust effect against colds in children (younger than 16 years of age) compared to adults. Cold duration decreased 21 percent among adults taking 2 grams daily and decreased 6 percent among adults taking only 1 gram daily. However, cold duration decreased 26 percent among children taking 2 grams daily and 17 percent among children taking only 1 gram daily.[23]
"Cold duration decreased 26% among children taking 2 grams of vitamin C daily and 17% among children taking only 1 gram of vitamin C daily." Click To Tweet
A meta-analysis of nine randomized controlled studies compared the administration of vitamin C as a prophylactic measure with or without therapeutic doses of vitamin C taken after the onset of symptoms to treat and prevent the common cold. The prophylactic regimens included doses between 1 and 3 grams of vitamin C taken per day over the course of several months along with a therapeutic dose of up to 6 grams at the onset of symptoms. The study found that prophylactic supplementation of vitamin C along with a therapeutic dose at the onset of common cold symptoms can reduce both the symptoms and the duration of a cold episode, while therapeutic doses given only at the onset of symptoms had no effect.[24] The analysis determined that taking vitamin C prophylactically and then increasing the dose when symptoms of a cold arise shortened the duration of a cold by half a day. If vitamin C was taken only at the onset of symptoms, there was no shortening of cold symptoms.
A meta-analysis of 29 trials involving more than 11,000 participants further evaluated the use of vitamin C as a prophylactic supplement at doses ranging from 200 milligrams to 2 grams per day for a time period of two weeks to six months. The analysis revealed that those who supplemented were only 4 percent less likely to develop a cold than those who did not supplement.[25] Some of the doses compared in this analysis were very low compared to other studies, so it is possible that higher doses could have prevented more colds, as demonstrated elsewhere.
Specialized needs of endurance athletes. These findings suggest that prophylactic vitamin C supplementation modestly but reliably reduces the risk of developing a common cold, possibly in a dose-dependent manner.[25] However, a subgroup of six trials involving more than 600 marathon runners, skiers, and soldiers reported 50 percent fewer colds, suggesting that people who frequently participate in high endurance exercise might be more responsive to supplemental vitamin C than others. This meta-analysis also compared adults and children in terms of the duration of colds that occurred among participants who were taking prophylactic doses. Among adults, 22 trial comparisons represented an 8 percent reduction in cold duration, compared to adults who did not take vitamin C. Among children, 12 trial comparisons represented a 13.5 percent reduction in cold duration, compared to those who did not consume vitamin C.[25]
Taken together, the current literature suggests that many factors, such as participants' age as well as supplement dose and duration, can affect the outcome of vitamin C supplementation on the incidence and duration of the common cold. Overall, vitamin C intake might be more effective in children and adolescents compared to adults. In addition, due to the short half-life of vitamin C – a few hours – and its limited bioavailability, 2 grams of oral intake taken in frequent doses throughout a 24-hour period saturate plasma vitamin C levels.[19] Finally, vitamin C supplementation is most effective against the common cold if taken prophylactically along with an increasing dose when symptoms arise.
However, many of the inconsistencies in the findings among the various trials investigating the efficacy of vitamin C against the common cold might be due to shortcomings in the trials' designs, especially those utilizing low and/or infrequent dosing schedules. But despite these widespread inadequacies, meta-analyses broadly demonstrate an effect, particularly with higher doses. Newer understandings of bioavailability might ultimately inform new trial designs that could show an even stronger effect, especially since plasma increases with low dose vitamin C treatments are both transient and diminished, especially in trials that don't establish rigorous, frequent dosing schedules. Ideal trial designs will assess blood kinetics based on dosing every few hours after symptom onset and utilizing larger doses of up to 3 grams.
Vitamin C and lung function
The innate immune system of the lungs plays an important role in the body's defense system as a means to protect the body from inhaled oxidants and pathogens. Vitamin C might help protect the lungs by boosting immune cell function and reducing oxidative stress.[26] Some evidence demonstrates that certain bacteria (including pneumococci) release large quantities of hydrogen peroxide into cells, inactivating inflammasomes and weakening the immune response to lung infection.[27] Inflammasomes are large, intracellular protein complexes that detect and respond to pathogens. Vitamin C's role as an antioxidant could have special relevance for lung pathogens by preventing them from inactivating the immune system. Multiple studies have investigated the use of vitamin C as a therapy to protect the lungs against oxidative stress caused by infections.
Vitamin C and the risk of respiratory diseases
Vitamin C might be protective against respiratory diseases, the fifth leading cause of death among people living in the United States. A population-based study analyzed the relationship between blood vitamin C concentrations and respiratory disease in more than 19,000 participants between the ages of 40 and 79 years without histories of respiratory diseases. To assess this relationship, the participants were separated into quartiles based on baseline blood vitamin C levels: less than 41, between 42 and 54, between 54 and 66, and greater than 66 micromoles per liter of blood. The study participants were followed for approximately 16.5 years. The analysis found that the participants with the highest blood vitamin C concentrations were 15 percent less likely to develop respiratory conditions and were 46 percent less likely to die of lung cancer, compared to the participants with the lowest blood vitamin C levels. The authors of the study noted that the participants with the highest baseline vitamin C levels also had lower risk factors for developing respiratory disease: they were generally younger and healthier, were less likely to smoke, consumed less alcohol, were more physically active, and had a lower prevalence of other chronic illnesses than those with lower vitamin C levels.[28]
Vitamin C and mechanical ventilation
Vitamin C has proven to be effective at decreasing the duration for which patients are kept on mechanical ventilation, an important strategy used to treat people experiencing respiratory failure. A meta-analysis of six studies analyzed the duration of mechanical ventilation among patients who received supplemental vitamin C versus those who did not. The analysis found a significant difference in the effect of vitamin C on the duration of mechanical ventilation between patients who were ventilated for more than 24 hours compared to those on ventilation for less than 24 hours. In three of those trials (two of which utilized intravenous vitamin C) patients who were ventilated for more than 24 hours and given vitamin C spent approximately 18 percent less time on mechanical ventilation compared to controls. However, in three other trials where patients were on ventilators for less than 24 hours neither intravenous nor oral vitamin C administration had an effect on the duration of ventilation.[29] These findings suggest that less than 24 hours on a ventilator is not sufficient time for vitamin C to exert protective effects.
Vitamin C and exercise-induced bronchoconstriction
Supplemental vitamin C might mitigate some of the symptoms associated with exercise-induced bronchoconstriction, demonstrating another way in which vitamin C might be beneficial for some groups of athletes, in addition to the effect on the common cold, as described above. Exercise-induced bronchoconstriction is a narrowing of the airways that occurs in response to exercise. It occurs in approximately 10 percent of the general population and up to 50 percent in some competitive athletes.[30]. The condition is characterized as a 10 percent or greater decline in exercise-induced forced expiratory volume, or FEV1 (a measure of respiratory capacity).[30] A meta-analysis of three placebo-controlled studies analyzed the relative exercise-induced decline in FEV1 among participants who took 0.5 to 2 grams of vitamin C versus a placebo. The participants were men and women between the ages of 7 and 28 years who took vitamin C immediately before exercise or every day for two weeks. The analysis found that vitamin C reduced the FEV1 decline by more than 8 percent post-exercise, indicating that vitamin C might alleviate respiratory symptoms caused by exercise.[31]
Vitamin C and pneumonia
Evidence suggests that vitamin C might be effective at preventing or treating pneumonia related to viral or bacterial lower respiratory infection. Pneumonia tends to be more severe among children under the age of 5 years and adults over the age of 65 years.(Source: NIH) Observational studies that investigated whether there was an association between dietary antioxidant intake and risk of community-acquired pneumonia did not find any association in well-nourished male and female health professionals living in the United States. [32] [33]
However, a study involving 50 patients with pneumonia and 50 healthy controls found that the patients with pneumonia had poor vitamin C status and elevated oxidative stress. Whereas average blood vitamin C levels among the healthy controls were 56 micromoles per liter, levels among the patients with pneumonia were 23 micromoles per liter. The pneumonia patients also exhibited elevated protein carbonyl concentrations compared with the healthy controls, indicating enhanced oxidative stress.[34] Furthermore, randomized controlled trials have found that vitamin C supplementation is protective against pneumonia in populations with low baseline vitamin C levels.
Over the past four decades, only five controlled studies have explored whether the use of vitamin C can lower the incidence or severity of pneumonia. Three of the studies investigated the use of vitamin C as a prophylactic treatment against pneumonia and each found a roughly 80 percent decrease in the incidence of pneumonia in participants who took vitamin C, compared to those who did not.[35] However, two of the studies that used vitamin C as a prophylactic involved people who were likely vitamin C deficient. In particular, one of the studies was conducted in a group of male students during World War II whose dietary vitamin C intake was very low (10 to 15 milligrams per day), while the other study was conducted in military recruits in the former Soviet Union.[36] [37]
A therapeutic trial involving 57 elderly patients diagnosed with either bronchitis or bronchopneumonia found that patients who took 200 milligrams of vitamin C per day showed improvements in major respiratory function compared to the patients who were not administered vitamin C.[38] These patients had baseline blood vitamin C levels of 23 micromoles per liter, far lower than optimal blood concentrations of 50 micromoles per liter. Taken together these studies suggest that vitamin C might decrease the incidence of pneumonia in people who have very low plasma levels of vitamin C.
Vitamin C and asthma
Asthma is a common long-term inflammatory disease of the airways of the lungs that affects a person's ability to breathe. Few studies have investigated the role of vitamin C in reducing the symptoms and severity of asthma attacks, and the findings have been mixed.
A study in 41 asthma patients (average age, 26 years) who took 1 gram of vitamin C or a placebo daily for 14 weeks found that the patients who took vitamin C had fewer, less severe asthma attacks compared to the placebo group.[39] A separate study gave 300 hundred asthma patients aged 18 to 60 years 1 gram of vitamin C and 450 milligrams of magnesium chelate or a placebo daily for 16 weeks. Low blood magnesium levels have been implicated in the severity of asthma.[40] The study identified no evidence of beneficial effects on any outcome measure of asthma control such as symptom scores, bronchodilator use, FEV1, and airway responsiveness to methacholine, a commonly used asthma drug.[41] More randomized placebo-controlled studies are needed to determine whether vitamin C might improve symptoms and reduce the severity of asthma attacks. Additional studies should determine if outcomes of asthma are altered by different doses of vitamin C, age demographics, or varying baseline levels of vitamin C
Vitamin C and lung cancer
Some evidence suggests that vitamin C might be protective against lung cancer, the second most common form of cancer among men and women living in the United States.(Source: American Cancer Society) A meta-analysis of 14 studies comprising more than 6,000 cases of lung cancer investigated whether a dose-response relationship played a role in vitamin C and reduced cancer risk. The findings indicated that for every 100-milligram increase in daily vitamin C intake among men, the risk of developing lung cancer decreased 7 percent. [42] In addition, as described above, a meta-analysis found that study participants with the highest blood vitamin C concentrations were 15 percent less likely to develop respiratory conditions and were 46 percent less likely to die of lung cancer, compared to study participants with the lowest blood vitamin C levels.[28]
"For every 100-milligram increase in daily vitamin C intake among men, the risk of developing lung cancer decreased 7 percent." Click To Tweet
Vitamin C and exercise
Vitamin C might enhance exercise performance by reducing the potential negative consequences of excess reactive oxygen species, while also blunting the beneficial training adaptations that reactive oxygen species might mediate. Reactive oxygen species are highly reactive molecules produced during normal metabolic processes as well as during exercise, as a consequence of exercise-induced immune activation.[43] Excessive exercise-induced reactive oxygen species can promote muscle damage, fatigue, and immune dysfunction, but the extent varies according to the duration and extent of exercise.[44] For example, high-intensity exercise such as long-distance running is linked to an increased incidence of upper respiratory infection.[45] However, reactive oxygen species might also mediate beneficial training adaptations as a part of a biologically useful signaling cascade.
Blunting of exercise-induced adaptations
Frequent exercise induces many beneficial adaptations in the body, such as increased mitochondrial number and function, improved insulin sensitivity and glucose utilization, and enhanced immune function, among others. Numerous studies have investigated the role that vitamin C supplementation has on exercise performance and physiological adaptations, with varying results. Supplemental vitamin C doses ranging from 400 milligrams to 3 grams per day decreased markers of muscle damage (such as creatine kinase activity and myoglobin concentrations) and reduced muscle soreness after exercise.[46] [47] [48]
However, findings from studies using lower supplemental doses between 200 milligrams and 1 gram per day suggest that vitamin C has no effect on physical adaptations to exercise.[46] [49] [50] Furthermore, vitamin C taken in combination with other antioxidants such as vitamin E appears to blunt cellular adaptations to exercise.[51] [52] For example, in a randomized controlled trial of 54 men and women (average age, 25 years) who took either 1 gram of vitamin C with 235 milligrams of vitamin E or a placebo for 11 weeks, both groups saw a similar improvement in VO2 max, compared to their pre-supplementation levels. However, markers of mitochondrial biogenesis decreased in participants taking vitamin C and E but increased in the placebo group.[52] These results indicate that supplementation of both vitamin C and E, particularly when combined, might attenuate beneficial cellular adaptations associated with exercise.
"Supplementation of both vitamin C and E, particularly when combined, might attenuate beneficial cellular adaptations associated with exercise." Click To Tweet
Multiple confounding factors are likely driving the variation in results from these studies. Such factors might be related to the dose and duration of vitamin C supplementation or whether vitamin C is taken alone or with other antioxidants. On the other hand, the study participants' level of physical fitness and baseline vitamin C levels might also contribute to the varying results. A meta-analysis of eight controlled trials with a supplement time course ranging from a few weeks to five months suggests that there might be an optimal dose of vitamin C that can reduce oxidative stress without impairing training adaptations. Furthermore, the analysis included studies that adopted a high-intensity maximal performance test, such as time to fatigue, along with supplementation that included vitamin C, either in isolation or mixed with other nutrients. It should be noted that four of the studies from the meta-analysis supplemented with vitamin C alone; three studies reported performance impairment, and one reported improvement, although all improvements were nonsignificant. The authors of the study concluded that vitamin C doses of 0.2 to 1 gram per day might reduce oxidative stress, while doses greater than 1 gram per day appear to impair beneficial adaptations to exercise.[53]
A separate study indicated that low vitamin C levels might be linked to decreased physical performance and increased oxidative stress. The study involved 100 men who were screened for baseline vitamin C blood levels. The 10 men having the lowest levels (approximately 35 micromoles per liter) and the 10 with the highest levels (approximately 78 micromoles per liter) were assigned to two groups. The 20 participants performed aerobic exercise to exhaustion before and after 30 days of supplementation with either 1 gram of vitamin C or a lactose control. At the end of the study, the participants with low vitamin C levels had lower VO2 max levels compared to the high vitamin C group. Blood biomarkers for oxidative stress decreased with vitamin C supplementation in the men with low baseline vitamin C levels only.[54]
One benefit of regular physical exercise is improved insulin sensitivity due to exercise-induced reactive oxygen species generation.[55] [56] Some studies suggest that vitamin C, when taken with other supplemental antioxidants, might attenuate these beneficial effects. For example, a study involving 40 men, 20 of whom were untrained and 20 pre-trained, found that 500 milligrams of vitamin C taken twice daily along with 400 IU of vitamin E reduced insulin sensitivity compared to placebo. All participants underwent an exercise training program of approximately one hour of cycling or running along with circuit training five days a week for four weeks along with daily supplementation. Physical exercise improved insulin sensitivity only in the absence of antioxidants.[57] However, another study in 21 physically active men between the ages of 18 and 40 years who were given 500 milligrams of vitamin C and 400 IU of vitamin E or a placebo every day for 16 weeks showed that antioxidant supplementation had no effect on insulin sensitivity.[58]
The differences between the findings of these studies might arise from the dose and duration of vitamin C supplementation in relation to exercise. In particular, the first study explored supplemental vitamin C taken at two separate occasions during the day, while the second study supplemented vitamin C at breakfast only, likely a few hours before exercise. Given that the half-life of oral vitamin C is a few hours, it is possible that sustaining vitamin C plasma levels by consuming multiple doses of vitamin C throughout the day can blunt exercise-mediated improvements in insulin sensitivity.
Taken together, the inconsistencies in the findings of studies investigating the effects of vitamin C on exercise-induced benefits might be attributable to differences in the conditions of vitamin C intake such as the dose, duration, and timing, as well as the exercise protocol. Another important variable is the addition of supplemental antioxidants (such as vitamin E) at doses that are considerably higher than the RDA. Furthermore, supplementation of vitamin C might affect exercise-induced oxidative stress and training adaptations differently depending on an individual's baseline vitamin C levels.
Vitamin C and exercise induced-immune function
Vitamin C has variable effects on exercise-induced immune function, one of the many beneficial physiological responses to exercise. Frequent, moderate to vigorous acute exercise sessions lasting around 60 minutes in length can enhance the body's immune system, but repeated high-intensity training or competition in endurance activities such as long-distance running is linked with immune dysfunction and increased illness due to exercise-induced oxidative stress.[44] In fact, epidemiological studies have linked endurance activities to a higher risk of upper respiratory infections.[59]
Studies have tested the use of vitamin C alone or in combination with vitamin E as a means to increase immune cell function and oxidative capacity to protect against exercise-induced immune dysfunction. Generally, supplemental vitamin C doses of 1 to 2 grams per day for one to two weeks rarely elicit improvements in immune function before or after prolonged exercise.[60] [61] [62] However, among those participating in high endurance training, vitamin C supplementation along with additional antioxidants such as vitamin E and beta-carotene might improve immune function. In addition, a meta-analysis of six trials (described above) involving more than 600 marathon runners, skiers, and soldiers reported 50 percent fewer colds, suggesting that people who frequently participate in high endurance exercise might be more responsive to supplemental vitamin C than others.[25]
A randomized controlled study in 20 athletes in their early twenties who participated in duathlon-like competitions found that antioxidant supplementation increased the antioxidant defenses of neutrophils. The athletes took either an antioxidant nutrient cocktail consisting of 250 milligrams of vitamin E and 15 milligrams of beta-carotene or a placebo composed of lactose every day for 90 days. During the final 15 days of the study, the athletes took an additional supplement of 1 gram of vitamin C or a placebo. At the end of the study period, plasma antioxidant concentrations were markedly higher among the supplemented group than those of the placebo group. The activity of catalase, superoxide dismutase, glutathione peroxidase, and glutathione reductase in the supplemented participants' neutrophils increased as well, compared to those who took the placebo, indicating enhanced neutrophil function.[63] Several other studies in trained runners have also indicated that vitamin C doses of 150 and 900 milligrams per day when supplemented in conjunction with vitamin E and beta-carotene, can enhance neutrophil function and reduce exercise-induced oxidative damage in lymphocytes.[64] [65] [66]
"Several studies in trained runners have indicated that vitamin C doses of 150 and 900 milligrams per day, when supplemented in conjunction with vitamin E and beta-carotene, can enhance neutrophil function and reduce exercise-induced oxidative damage in lymphocytes." Click To Tweet
Vitamin C and fatty acid oxidation
Vitamin C levels are inversely related to body weight, particularly among people who are obese, as described above.[67] [68] Given that vitamin C is essential for the synthesis of carnitine, a compound required for the utilization of fatty acids as energy, it is possible that low vitamin C levels contribute to increased fat storage. An in vivo study in mice that were fed an obesogenic diet (high fat and high sugar) with or without vitamin C found that vitamin C reduced body weight gain, particularly in terms of fat mass, compared to mice fed the obesogenic diet alone.[69] In addition, a mouse model of Werner syndrome – a genetic disorder characterized by premature aging – demonstrated that vitamin C supplementation extended the mean lifespan of the mice and corrected many of the age-related metabolic diseases characterized as metabolic syndrome. The supplemented mice also exhibited increased levels of PPAR-alpha, a protein that regulates fat metabolism by increasing uptake and utilization.[70]
"An in vivo study in mice fed an obesogenic diet (high fat and high sugar) with or without vitamin C found that vitamin C reduced body weight gain, particularly in terms of fat mass, compared to mice fed the obesogenic diet alone." Click To Tweet
A clinical study in 22 men and women between the ages of 18 and 38 years evaluated the relationship between vitamin C status and fat oxidation during exercise. Fifteen of the participants had marginal vitamin C blood levels (less than 34 micromoles per liter) and seven had adequate vitamin C blood levels (greater than 34 micromoles per liter). All of the participants completed a 60-minute treadmill walk at 50 percent of their VO2 max. Fat utilization during exercise was 25 percent lower among participants with marginal vitamin C status, suggesting that vitamin C status affects fuel utilization during exercise. Furthermore, after four weeks of vitamin C depletion, participants took either 500 milligrams of vitamin C or a placebo every day for four additional weeks. At the end of the eighth week, average blood vitamin C levels in the supplemented group were approximately 42 micromoles per liter, versus approximately 10 micromoles per liter in the depleted group. Additionally, fatty acid utilization in the supplemented group was approximately four times greater than in the vitamin C depleted group.[71] The animal and clinical studies described here indicate that vitamin C status participates in fat metabolism, and adequate vitamin C levels might be necessary for effective fat utilization and weight management.
Vitamin C and the brain
Vitamin C is found in high concentrations in the brain, especially in the hippocampus and frontal cortex regions.[72] [73] Evidence suggests that vitamin C might play an important role in brain development and serve as a defender against reactive oxygen species-induced neurodegeneration.
Multiple animal studies have demonstrated the importance of vitamin C in brain development. In mouse models that lack SVCT2 (a cellular transporter for vitamin C), mice exhibit increased oxidative damage in their brains and do not survive birth due to respiratory failure and brain hemorrhages.[74] [75] Studies in newborn guinea pigs have demonstrated that prenatal and postnatal vitamin C deficiency stunted hippocampal development in newborn guinea pigs by 10 to 30 percent.[76][77] Similarly, studies in human fetuses and stillborn babies suggest an important role for vitamin C in brain development during gestation. Particularly, vitamin C levels were up to 11 times higher at 11 weeks gestation compared to adults. Compared to adults, full-term babies (37 to 42 weeks gestational age) had at least three times higher vitamin C levels.[78] [79]
Vitamin C and neurodegenerative disorders
Vitamin C's antioxidant capacity might be beneficial in decreasing the risk of neurodegenerative disease by reducing oxidative damage. Neurodegenerative disorders comprise a broad range of diseases caused by the progressive death of neurons in the central and peripheral nervous systems. Common neurodegenerative diseases include Alzheimer's disease, Parkinson's disease, Huntington's disease, and multiple sclerosis. Several factors drive neurodegeneration, but oxidative stress likely mediates and enhances the processes involved.[80] Two observational studies suggested that Alzheimer's disease is accompanied by decreased antioxidant status and increased oxidative stress, indicating that oxidative damage might be one underlying cause of neurodegeneration.[81] [82] An observational study in 72 patients, 53 of whom were diagnosed with Alzheimer's disease and 19 who were not, found that despite similar intakes of vitamin C, blood concentrations of vitamin C decreased in proportion to the severity of the cognitive impairment.[83]
Furthermore, vitamin C intake might protect against dementia and cognitive decline. [84] [85] For example, scientists in the Netherlands conducted a 10-year-long study involving nearly 5,400 participants who were at least 55 years of age to determine whether dietary intake of antioxidants was related to the risk of developing Alzheimer's disease. After a mean follow-up of six years, 146 of the participants had been diagnosed with Alzheimer's disease, and the average intake of vitamin C was approximately 122 milligrams per day. For every 54-milligram increase in vitamin C intake, there was an 18 percent reduction in the risk of Alzheimer's disease. In smokers, the protective effect of vitamin C was even more robust with a 35 percent decrease in Alzheimer's disease risk for every 54-milligram increase in vitamin C.[86]
"For every 54-milligram increase in vitamin C intake, there was an 18% reduction in the risk of Alzheimer's disease. In smokers, the protective effect of vitamin C was even more robust with a 35% decrease in Alzheimer's disease risk for every 54-milligram increase in vitamin C." Click To Tweet
Vitamin C and functions in the brain
Evidence suggests that vitamin C protects the brain from oxidative damage derived from high rates of cellular metabolism. Aside from the antioxidant role of vitamin C in the brain, vitamin C has also been implicated in both neuromodulation and brain development. Specifically, vitamin C serves as a cofactor in the regulation of neurotransmitters by preventing excess dopamine accumulation in neurons that generate reactive oxygen species.[87] In vitro studies suggest that vitamin C might also aid in the formation of neural circuits.[88] [89] Furthermore, vitamin C is an important compound needed for the regulation of hypoxia-inducible factor 1-alpha, or HIF 1-alpha, a transcription factor that has been implicated in the pathogenesis of cancer and other diseases.[90] [91]
Intravenous Vitamin C and its use for the treatment of infection
For the past several decades, intravenous vitamin C has been used as a treatment for multiple types of viral infections.[92] Intravenous vitamin C's effectiveness as an antiviral treatment is likely due to its immune system-enhancing capabilities. In addition, some studies have observed that in critically ill patients such as those with viral infections, sepsis, or accidental injury, plasma levels of vitamin C might be less than 25 percent of those observed in healthy people.[93] [94] [95]
Intravenous Vitamin C for the treatment of sepsis
Sepsis, a life-threatening condition that can arise due to the body's response to a bacterial or viral infection, can cause severe injury to multiple tissues or organs.(Source: CDC) People diagnosed with sepsis typically have low vitamin C levels, which might be predictive of increased risk for organ failure.[95] Evidence suggests intravenous vitamin C might be an effective treatment for sepsis. For example, experimental studies in mice have demonstrated that intravenous vitamin C attenuates the proinflammatory state associated with sepsis and reduces biomarkers of organ injury to promote the preservation of organ function, compared to mice not treated with vitamin C.[96] [97] [98]
In clinical studies, intravenous vitamin C reduced the number of hospital deaths among patients with sepsis. One study involved more than 160 patients admitted to the intensive care unit for the treatment of sepsis-induced acute respiratory failure. The patients were randomized to receive a placebo or intravenous vitamin C at 50 milligrams per kilogram of body weight (mg/kg/bw) every six hours for 96 hours. The study revealed no differences in the primary outcomes of organ failure, inflammation, or vascular injury. However, 28 days after the beginning of the study, approximately 46 percent of the patients in the placebo group died, compared to less than 30 percent in the intravenous vitamin C-treated group. Furthermore, patients in the vitamin C-treated group had an increased number of ventilator-free days, were transferred out of the intensive care unit at a higher rate, and had an average increase of seven hospital-free days compared to the placebo group.[99]
A separate study treated 47 sepsis patients with 6 grams of intravenous vitamin C four times per day for four days along with hydrocortisone (a type of steroid medication) and thiamin (a type of B vitamin). A control group of 47 other sepsis patients received the standard of care. Approximately 40 percent of the patients in the control group died, but approximately 8 percent of the patients in the treated group died. In addition, the treated group exhibited improved organ function compared to the control group.[100] These studies suggest that intravenous vitamin C alone or in combination with other treatments decreases the risk of organ failure and death in patients diagnosed with sepsis.
Intravenous vitamin C for the treatment of myocarditis in children
Myocarditis is inflammation of the heart muscle commonly caused by viruses. Symptoms include shortness of breath, chest pain, and heart failure.[101] A meta-analysis found that intravenous vitamin C combined with conventional therapy is better than the conventional therapy alone for the treatment of viral myocarditis in children. The analysis compared data from eight studies of more than 420 children administered conventional therapy and intravenous vitamin C doses between 100 and 250 mg/kg/bw. A control group of more than 360 children received conventional therapy alone. Outcome indicators included measures of lactate dehydrogenase, creatine kinase, and creatine kinase isoenzyme, enzymes for which rapid increases are associated with myocardial ischemia and lesions – key clinical indicators of myocarditis. Overall, conventional therapy in combination with intravenous vitamin C decreased all enzymes, compared to conventional therapy alone.[102]
Intravenous vitamin C for the treatment of herpes
Although intravenous vitamin C is widely acclaimed as a successful treatment for patients with herpes simplex virus (the cause of oral and genital herpes), there are currently no clinical studies supporting these claims. However, multiple case studies and observational reports suggest a role for intravenous vitamin C in reducing pain in patients with varicella-zoster virus (the cause of chickenpox) and herpes zoster (the cause of shingles). A study involving 38 patients with postherpetic neuralgia (severe pain that persists after a herpes zoster outbreak) and 39 healthy volunteers, blood vitamin C levels were approximately 66 percent lower among the patients compared to the volunteers.[103] Furthermore, in a study of 41 patients who received three infusions of 2.5 grams of intravenous vitamin C or a placebo over a period of five days, those who received the infusions reported having less pain compared to the control group.[103]
A separate randomized study assessed pain in nearly 90 patients who were administered saline with or without 5 grams of vitamin C for three days. After two weeks of follow-up, the patients' pain assessments were modestly lower than the control group, suggesting that pain might be more effectively controlled over time with intravenous vitamin C. Individual case reports support the use of intravenous vitamin C as a means to decrease pain among patients with this condition.[104] [105]
Intravenous vitamin C for the treatment of Epstein-Barr virus infection
Epstein-Barr virus is a type of herpes virus that causes mononucleosis. A retrospective study – a study that compares groups of individuals over a period of time in the past who share a common disease exposure – found that patients who were infected with Epstein-Barr virus and were administered intravenous vitamin C exhibited decreased viral infection and replication. The study, which involved more than 175 Epstein-Barr patients who were treated with 7.5 to 50 grams of intravenous vitamin C for varying durations, found that antibody levels decreased 42 percent, compared to levels before treatment.[106] This decrease in antibody levels might be a result of hindering viral infection and replication as observed in _ in vitro_ studies.[107] [108] Although these findings seem promising, more randomized controlled studies are needed to determine the extent to which intravenous vitamin C might be used as a therapy for mononucleosis.
Intravenous Vitamin C and cancer
Intravenous vitamin C as adjunctive treatment to chemotherapy
In 1976, Nobel Prize-winning chemist Linus Pauling found clinical evidence suggesting that high dose intravenous administration of vitamin C is useful as a supportive treatment for cancer as well as a method to mitigate the side effects of chemotherapy.[109] Chemotherapy is a pharmacological method used to cure cancer or control its spread. Although chemotherapy treatments are used to kill fast-growing cells such as cancer, they can also kill healthy cells, such as blood cells or hair follicles, causing many unwanted side effects. Pauling's work demonstrated that terminal cancer patients – those for whom conventional treatment would offer no further benefit – who received vitamin C infusions lived longer than patients who did not. His study found that terminal cancer patients with various types of terminal cancers who were given 10 grams of vitamin C via intravenous infusion for 10 days, followed by 10 grams of oral vitamin C per day indefinitely, lived more than 210 days longer than terminal cancer patients who did not receive vitamin C.[110]
Although Pauling's study was heavily criticized due to lack of proper controls and standardization, many clinical studies have since suggested a beneficial use for intravenous vitamin C as adjunctive therapy for cancer. For example, the efficacy of intravenous vitamin C has been tested in patients diagnosed with various types of cancers, including glioblastoma, melanoma, and pancreatic, ovarian, colorectal, and non-small cell lung cancer, with doses ranging from 500 milligrams daily to 100 grams three times per week.[109] (Source: National Cancer Institute) [111]
The findings from many of these studies suggest a trend in the overall survival rate for patients administered intravenous vitamin C in conjunction with standard therapy.[112] For example, in a recent phase 1 clinical trial, 13 patients diagnosed with glioblastoma were treated with radiation and chemotherapy along with 15 to 125 grams of intravenous vitamin C three times per week. The patients' overall median survival (length of time from the date of diagnosis in which the patients are still alive) was 18.2 months compared to the historical median survival rate of 14 months for patients with this disease.[113] (Source: ClinicalTrials.gov Institute) Similar results were reported in a case study of a woman who, after being diagnosed with glioblastoma and taking 85 grams of intravenous vitamin C three times per week for six months, followed by twice per week for approximately three years, survived more than four years.[114]
Two separate studies in patients diagnosed with pancreatic cancer demonstrated that intravenous vitamin C can reduce tumor size and promote a trend toward longer progression-free survival – the duration of time during and after the treatment of a disease in which the disease does not get worse – or overall survival, compared to standard chemotherapy alone. The first trial was a dose-escalation study in which nine patients diagnosed with pancreatic cancer received 50, 75, or 100 grams of intravenous vitamin C three times a week for eight weeks along with the standard treatment of gemcitabine and erlotinib. At the end of the study period, eight of the nine patients exhibited reduced tumor size, compared to tumor size at the start of the trial. In addition, the mean progression-free survival was nearly 13 weeks compared to the mean progression-free survival of nine weeks for patients who took gemcitabine alone.[115] The second study involved nine patients diagnosed with pancreatic cancer who received intravenous vitamin C doses between 50 and 125 grams twice per week, in conjunction with the standard treatment gemcitabine, for an average treatment duration of six months. The patients' mean progression-free survival was 26 weeks and their overall survival was 12 months, compared to six months mean overall survival for patients taking gemcitabine alone.[115] [116]
Furthermore, a randomized controlled trial involving patients diagnosed with stage III and stage IV ovarian cancer showed that intravenous vitamin C promoted a trend toward an increase in median overall survival compared to patients who did not receive vitamin C. In this study, 13 patients received the chemotherapy treatment of carboplatin and paclitaxel along with varying doses of intravenous vitamin C, ranging from 15 to 100 grams per infusion twice per week. A control group of 12 patients received chemotherapy alone. After six months of chemotherapy, the patients who received intravenous vitamin C continued to take the same dose of vitamin C for an additional six months. The median time for disease progression and relapse was nearly nine months longer for patients taking chemotherapy with vitamin C compared to the control group.[117]
These results have not been recapitulated in cancer patients who have been administered high oral doses of vitamin C. Two randomized, double-blind, placebo-controlled studies in which 200 terminal cancer patients received 10 grams of oral vitamin C once a day demonstrated no benefit.[118] [119] These inconsistencies are likely due to the differences in absorption and bioavailability between oral and intravenous vitamin C (as described above).
Intravenous vitamin C and quality of life in patients with cancer
Intravenous vitamin C also demonstrates efficacy in improving cancer patients' quality of life outcomes. A study involving 39 patients diagnosed with terminal cancer who received 10 grams of intravenous vitamin C twice separated by a three-day interval and 4 grams of oral vitamin C daily for a week found that patients reported improvements in physical, emotional, and cognitive status as well as reduced fatigue, nausea, vomiting, pain, and appetite loss after intravenous vitamin C.[120] Another observational study of 125 patients diagnosed with breast cancer, 53 of whom received 7.5 grams of intravenous vitamin C once per week in conjunction with their standard chemotherapy and radiation therapies, noted improved quality of life compared to patients who only received chemotherapy and radiation.[121] Although these studies are purely observational, other clinical studies have indicated that intravenous vitamin C, when taken with standard chemotherapies, might improve the quality of life for patients.[117] [122]
"A study involving patients with terminal cancer found that intravenous and oral vitamin C improved physical, emotional ,and cognitive status as well as reduced fatigue, nausea, vomiting, pain, and appetite loss." Click To Tweet
While multiple clinical studies have suggested that intravenous vitamin C might be beneficial as an adjunct to standard chemotherapy, it is noteworthy that many of the clinical studies published were observational or had no direct control group. Furthermore, case studies in multiple patients have identified no evidence of improved survival or quality of life. [123] Nevertheless, intravenous vitamin C might be promising as adjunctive therapy for various types of cancer. Ongoing and future research will likely determine specific types and stages of cancer along with an appropriate chemotherapy regimen that would call for the use of vitamin C.
Effect of vitamin C on fertility and reproduction
Infertility affects as many as 180 million people worldwide.[124] [125] One of the contributors to male infertility is excessive reactive oxygen species.[126] [127] [126] Evidence suggests that vitamin C decreases reactive oxygen species and increases fertility due to its antioxidant activity. A study in 13 infertile men between the ages of 25 and 35 years who took 1 gram of oral vitamin C twice daily for two months demonstrated that the men's sperm count increased 58 percent and sperm motility increased 48 percent, compared to baseline measurements taken before supplementation, indicating improved semen quality.[128] Furthermore, 1 gram of oral vitamin C increased sperm quality in men exposed to toxins such as lead or those found in cigarette smoke.[129]
"A study in infertile men who took 1 gram of oral vitamin C twice daily for two months demonstrated that the men's sperm count increased 58 percent and sperm motility increased 48 percent, indicating improved semen quality." Click To Tweet
Intravenous vitamin C as a strategy to lower blood pressure
High blood pressure is a major risk factor for heart disease and stroke, two of the leading causes of death among people living in the United States.(Source: CDC) Large, population-based studies have found that vitamin C might confer protection against high blood pressure.[130] A meta-analysis of 29 randomized placebo-controlled clinical trials involving more than 1,400 participants demonstrated that patients with hypertension who took an average of 500 milligrams of oral vitamin C per day for approximately eight weeks exhibited significant decreases in blood pressure compared to those who took a placebo. [131]
Recent research suggests that intravenous vitamin C can acutely reduce blood pressure in patients diagnosed with prehypertension, a condition characterized by blood pressure between 120/80 mmHg and 139/89 mmHg – a risk factor for hypertension. A study involving 26 patients (average age, 54 years) who received 15 to 100 grams of intravenous vitamin C over the course of 80 minutes measured blood pressure at baseline and every 10 minutes during treatment. The results indicated that vitamin C infusion reduced blood pressure up to 10 to 15 mmHg in the first 10 to 20 minutes but blood pressures returned to baseline by 30 minutes. The authors of the study suggested that this might have been due to the physiological effects of the infusion itself, a phenomenon known as venodilation. However, by 75 minutes of infusion, intravenous vitamin C administered at doses above 30 grams significantly reduced systolic blood pressure up to 6 mmHg and diastolic blood pressure up to approximately 7 mmHg, when compared to blood pressure pre-infusion, likely due to the effects of the vitamin C.[132] These results were reversed when intravenous vitamin C was administered along with vitamin B12, however. While intravenous vitamin C shows promise in acutely reducing blood pressure, more randomized placebo-controlled studies are needed to determine the degree to which intravenous vitamin C is effective as well as the duration of its effects.
Intravenous vitamin C as a treatment for heart attack-associated ischemia and reperfusion injury
Ischemia is characterized by the restriction of blood supply to a tissue. Many conditions can cause ischemia, including atherosclerosis and cardiac arrest. When blood supply returns to the tissue, reperfusion injury can occur, promoting an inflammatory response accompanied by oxidative damage to the tissue. Vitamin C might be effective at reducing myocardial injury in part by mitigating oxidative stress. Two clinical studies using intravenous vitamin C have been conducted in patients undergoing a percutaneous coronary intervention, or PCI, a non-surgical procedure used to open blocked or narrowed arteries in the heart. A PCI is commonly performed after myocardial infarction, a primary risk factor for reperfusion injury. The studies found that intravenous vitamin C administration can reduce oxidative stress and reduce the occurrence of reperfusion-induced myocardial injury.[133] [134] One study randomized more than 560 patients into two groups, one of which received 3 grams of intravenous vitamin C within six hours of PCI, and the other of which received intravenous saline as a control. Markers of myocardial injury were decreased among the patients who received the intravenous vitamin C, compared to the controls, suggesting that intravenous vitamin C is associated with less myocardial injury.[135]
Ischemia and reperfusion injury can also occur in the brain following a stroke, causing increased inflammation and oxidative damage. Few clinical studies have been performed investigating the effects of intravenous vitamin C in mitigating ischemia in the brain. One placebo-controlled trial gave 30 patients with ischemic stroke 500 milligrams of intravenous vitamin C every day for 10 days, starting one day after the stroke occurred. A control group received the normal standard of care. No differences were observed in the clinical status of either group of patients using standard assessments of neurological status during the 10 days of treatment or after three months post-treatment.[136] While it's possible that intravenous vitamin C might not be effective after ischemic stroke when oxidative damage has already occurred, another possible explanation for the lack of efficacy observed in this study was the low dose used, since intravenous vitamin C doses are typically administered in gram quantities.
Vitamin C and inflammation
Inflammation is a biological response triggered by the immune system in response to a physical injury or infection. Vitamin C's immune-boosting and antioxidant properties mediate the body's inflammatory response, reducing the symptoms or risk of various diseases.
A randomized study of nearly 400 healthy adults (average age, 44 years) who took 1 gram of vitamin C, 800 international units of vitamin E, or a placebo every day for two months investigated whether antioxidants could lower C-reactive protein, or CRP, an inflammatory biomarker associated with cardiovascular disease risk. Blood levels of CRP greater than 1 milligram per liter are indicative of elevated cardiovascular disease risk. The study found that vitamin E had no effect on lowering CRP; however, vitamin C supplementation decreased CRP by nearly 17 percent compared to pre-treatment measurements, but only in participants who had baseline CRP levels above 1 milligram per liter. Among those who took the vitamin C supplement, median CRP levels decreased by more than 25 percent compared to the placebo group.[137] Furthermore, some studies have demonstrated that statins – a class of drugs used to lower blood lipids and decrease the risk of cardiovascular disease – can reduce CRP levels by as much as 17 percent.[138] [139] [140] Although more studies are needed, vitamin C might be able to decrease inflammation as well as some statins in people who are at greater risk of cardiovascular disease based on CRP levels.
Rheumatoid arthritis is an autoimmune disorder characterized by widespread systemic inflammation.[141] Some studies have tested the effect of intravenous vitamin C in reducing inflammation and pain in patients with rheumatoid arthritis due to its capacity to modulate the immune system and decrease oxidative stress. An analysis of 11 women between the ages of 45 and 69 years who were diagnosed with rheumatoid arthritis and had been given 7.5, 15, or 25 grams of intravenous vitamin C for varying durations found that the women's CRP levels decreased 44 percent compared to levels before treatment.[142] While more studies are needed to determine if decreasing CRP in patients with arthritis leads to improvements in joint and bone health, vitamin C might be useful in decreasing some aspects of inflammation.
Vitamin C's mechanisms of action
Immune cells actively participate in eliminating pathogens such as bacteria or viruses from the body. Vitamin C is highly concentrated in immune cells, with neutrophils and leukocytes having 50 to 100 times higher vitamin C concentrations than plasma, where it serves as a potent antioxidant.[143] [144]
One of the early stages of the body's immune response to viral or bacterial infection involves neutrophil infiltration into an affected tissue, where the cells engulf the pathogens and initiate their removal. Neutrophils also generate large quantities of reactive oxygen species as a part of an "oxidative burst" that is important to their function. The high levels of vitamin C present in immune cells protect them from reactive oxygen species-induced DNA damage while also promoting neutrophil reactive oxygen species production.[145] [64] In addition, studies in guinea pigs suggest that vitamin C plays an important role in facilitating neutrophil migration to sites of infection.[146] [147] Furthermore, studies in humans have also shown that vitamin C supplementation can enhance neutrophil function in young men between the ages of 18 and 30 years as well as in older women (average age, 72 years).[148] [149]
Vitamin C also appears to boost the immune system by promoting the proliferation of T cells and preventing T cell death. T cells play a major role in driving an immune response against pathogens such as bacteria or viruses. Multiple in vitro studies in both mouse and human cell lines have demonstrated that growing T cells in culture with vitamin C decreases cell death and might enhance T cell development.[150] [151] [152] [153] [154]
Aside from enhancing immune cell function, vitamin C modulates cytokine levels in _ in vivo_ studies. Cytokines, a class of proteins secreted by many types of immune cells, are important signaling molecules produced in response to inflammation and infection. In mouse models in which mice are vitamin C-deficient and have increased pro-inflammatory cytokines, the administration of vitamin C decreased cytokine production.[155] [156] Furthermore, vitamin C participates in the production of interferon in mice. Interferon is a type of cytokine that signals the body to initiate antiviral defenses.[155]
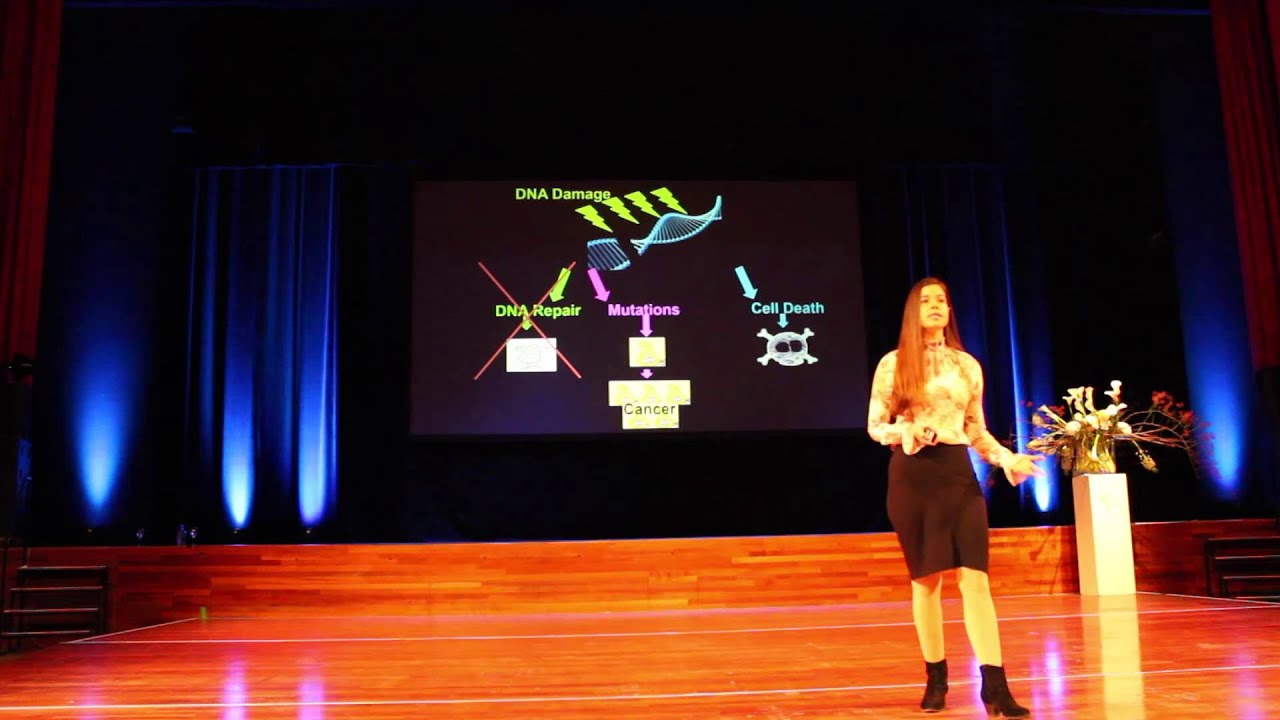
Intravenous vitamin C's mechanisms of action in infections and cancer
Vitamin C's protective capacity against some types of infections and cancers is likely attributable to multiple overlapping discrete mechanisms such as blocking glucose uptake, inhibiting viral replication, and generating hydrogen peroxide.
Most cancers rely primarily on glucose to generate energy through the process of glycolysis. Consequently, many types of cancers demonstrate increased glucose uptake.[157] As described above, vitamin C is transported across cellular membranes by sodium-dependent vitamin C transporters and glucose transporters.[158] [159] The glucose transporters primarily import dehydroascorbic acid, the oxidized form of vitamin C, into cells.[160] [161] An _ in vitro_ study suggested that the import of dehydroascorbic acid (the oxidized form of vitamin C) into cancer cells can cause oxidative stress and decrease cancer cell viability.[162] Furthermore, some of the glucose transporters have a greater propensity to transport dehydroascorbic acid into cells over glucose. [163] Therefore, high concentrations of dehydroascorbic acid derived from vitamin C might inhibit glucose uptake into cancer cells and therefore hinder cancer cell fuel utilization needed for survival and growth.
While vitamin C acts primarily as an antioxidant at physiological concentrations of approximately 50 micromoles per liter, pharmacologic doses of intravenous vitamin C greater than 1 gram generate hydrogen peroxide, a type of reactive oxygen species that can damage DNA, RNA, and proteins, leading to tissue damage.[164] Multiple _ in vitro_ and _ in vivo_ studies suggest that high dose vitamin C can assist in cancer cell death primarily due to the formation of hydrogen peroxide.[165] [166] [167] Some evidence suggests that vitamin C generates hydrogen peroxide by interacting with high levels of iron found in cancer cells.[168] Cancer cells often have lower levels of enzymes involved in the detoxification of reactive oxygen species compared to healthy cells, potentially increasing oxidative stress and causing cell death in cancer cells.[169] Successive treatments with high-dose intravenous vitamin C do not increase pro-oxidative markers in healthy people, suggesting that normal cells are not damaged by the burst of hydrogen peroxide produced by the intravenous vitamin C.[170]
Vitamin C also interferes with the replication of viral particles. One in vitro study showed that human immunodeficiency virus type 1-infected cells that were incubated for four days with 150 micrograms per milliliter of vitamin C decreased reverse transcriptase activity by 99 percent and p24 antigen levels by 13 percent (parameters of virus production) compared to untreated cells.[171] Another in vitro study demonstrated a dose-dependent effect of vitamin C to kill influenza viruses, with a vitamin C concentration of 2.5 millimoles per liter eliminating 90 percent of the virus present and a concentration of 20 millimoles per liter fully impeding viral replication.[107]
Furthermore, a study using a vitamin C-deficient mouse model demonstrated that these mice had increased pro-inflammatory cytokines and a 10- to 15-fold increase in viral titers in their lungs during viral infection compared to normal mice. One week after the mice developed a viral infection, all of the vitamin C deficient mice had died, but all the normal mice survived. When the vitamin C-deficient mice were supplemented with vitamin C prior to viral infection to achieve plasma levels of approximately 80 to 100 micromoles (similar to that of normal mice), there was no increase in viral titers in their lungs, but the production of antiviral cytokines increased. Supplementation with vitamin C prior to viral infection protected the formerly deficient mice from death.[155]
Vitamin C safety
Oral vitamin C
Vitamin C intakes at doses above the recommended dietary allowance for oral vitamin C are safe and well-tolerated in most people.[172] [173] As described above, a tolerable upper intake level of 2,000 milligrams of oral vitamin C has been established to reduce potential side effects associated with gastrointestinal upset, such as diarrhea.
Vitamin C supplementation is contraindicated in some medical conditions, however. For example, hemochromatosis is a condition that causes the body to absorb excessive iron from the diet, increasing blood iron levels. The primary treatments for hemochromatosis involve phlebotomy (the removal of blood via the veins) and iron chelators (medications that bind iron so it can be eliminated from the body). Vitamin C increases intestinal absorption of iron, so patients undergoing phlebotomy should avoid all dietary and supplemental sources of vitamin C. People who take iron chelators should not take more than 200 milligrams of vitamin C daily.[174] Iron levels can be high in otherwise healthy people, too, however. People whose iron levels are abnormally high should exercise caution when considering vitamin C supplementation.
Intravenous vitamin C safety
Intravenous vitamin C is well tolerated and has low toxicity.[122] [175] [115] [176]. The most commonly reported side effects include mild to moderate nausea, headache, and dry mouth, while less commonly reported side effects include fatigue, hypertension, loss of appetite, and hyperglycemia.[109] [177] Some serious side effects have been reported with high dose intravenous vitamin C in patients with cancer. In addition, people who have a deficiency in the enzyme glucose-6-phosphate dehydrogenase are at risk of hemolysis – the rupturing of red blood cells – when given high doses of vitamin C.[178] [179] [180] Although these studies suggest that vitamin C should be contraindicated in these conditions, the intravenous doses administered were 40 grams or higher. Other case reports have indicated that when given at a dose between 1 and 10 grams, intravenous vitamin C can reduce hemolysis.[181] [182] [183] Therefore, it is possible that at lower doses intravenous vitamin C is safe in people with glucose-6-phosphate dehydrogenase deficiency, but patients should exercise caution and be pre-screened for this deficiency before receiving high dose intravenous vitamin C.[184]
Vitamin C intake and kidney stone risk
Some studies have suggested that vitamin C intake is a risk factor for kidney stones due to the formation of oxalate, an end product of vitamin C metabolism.[185] The kidneys typically filter oxalate and excrete it in urine, but when high amounts of oxalate are present, it can form crystal structures with calcium. These crystal structures can lead to the formation of solid masses that can promote the formation of kidney stones.(Source: National Kidney Foundation) [186] A small number of case reports indicate that oxalate nephropathy, a condition in which oxalate calcium crystals form in the kidney, has been observed in patients with kidney impairment who have been administered high dose intravenous vitamin C.[187] [188] [189] Two large prospective cohort studies involving tens of thousands of people found that high vitamin C intake increases the relative risk of kidney stones.[190] [191] However, the findings from these studies are slightly misleading due to the fact that they did not report the actual incidence rate of kidney stones. It is worth noting that this risk was only found after statistical manipulation of the data and that the patients with higher vitamin C actually did not have more kidney stones.
Another prospective cohort study in more than 48,000 Swedish men found that the men's risk of developing kidney stones was 0.16 percent per year among men who did not supplement, but the risk was 0.30 percent per year among those who did, a statistical difference with no clinical significance.[192] In addition, the actual incidence rate was extremely low. In other words, those who supplement with vitamin C can expect a kidney stone every 323 years whereas those who do not supplement with vitamin C can expect a kidney stone every 613 years. Other studies have reported no impairments in kidney function with high dose intravenous vitamin C in cancer patients.[193] [176] Taken together these studies suggest that high dose vitamin C might lead to kidney stones in patients with preexisting kidney impairments, but vitamin C poses a relatively low risk of developing kidney stones in most healthy people.
Conclusion
Widely recognized as a potent antioxidant and a critical component of the immune system, vitamin C participates in myriad biological processes and pathways and affects multiple organ systems. Recommended intakes for vitamin C vary according to age, sex, and life stages of healthy people, but higher intakes, especially in quantities achieved with supplemental vitamin C, are associated with reduced risk of developing a vast array of acute and chronic diseases, ranging in severity from the common cold to cancer, cardiovascular disease, and neurodegenerative disorders. Conversely, low vitamin C intake, which varies across different populations, and subsequent deficiencies impair key biological processes and pose an increased risk for certain conditions such as decreased fat utilization during exercise and increased severity of Alzheimer's disease. Vitamin C might be especially beneficial for critically ill people, particularly those with viral infections, who commonly have lower blood levels of vitamin C compared to healthy people.
The effects of intravenous vitamin C differ markedly from those achieved with dietary or supplemental intake because of the considerable concentrated increase in plasma levels that occurs with intravenous administration. Consequently, intravenous vitamin C offers promise as a therapeutic strategy against certain types of cancer and infections that oral supplementation cannot achieve. With some exceptions, oral and intravenous vitamin C supplementation have been shown to be safe, well-tolerated, and have low toxicity.
The seemingly contradictory findings from much of the research on vitamin C arise from differences in study design, populations, dose, and delivery modalities, as well as a host of other factors. Future studies based on consistent, equivocal study designs are necessary to elucidate the full potential of vitamin C in benefiting human health.
- ^ Degré, Miklos; Dahl, Helen (2009). The Effect Of Ascorbic Acid On Production Of Human Interferon And The Antiviral Activity In Vitro Acta Pathologica Microbiologica Scandinavica Section B Microbiology 84B, 5.
- ^ 10.3164/jcbn.10-142
- ^ Levine, Mark (1999). Criteria And Recommendations For Vitamin C Intake Jama 281, 15.
- ^ a b Panel On Dietary Antioxidants And Related Compounds; Subcommittee On Upper Reference Levels Of Nutrients; Subcommittee On Interpretation And Uses Of Dietary Reference Intakes; Standing Committee On The Scientific Evaluation Of Dietary Reference Intakes; Food And Nutrition Board; Institute Of Medicine (2000). Dietary Reference Intakes For Vitamin C, Vitamin E, Selenium, And Carotenoids , .
- ^ Schectman, G; Byrd, J C; Hoffmann, R (1991). Ascorbic Acid Requirements For Smokers: Analysis Of A Population Survey The American Journal Of Clinical Nutrition 53, 6.
- ^ Faizallah R; Morris AI; Krasner N; Walker RJ (1986). Alcohol enhances vitamin C excretion in the urine. Alcohol Alcohol 21, 1.
- ^ Valentini, Luzia; Hengstermann, Susanne; Schaper, Lennart; Buning, Carsten; Koernicke, Thomas; Maritschnegg, Michaela, et al. (2008). Altered Status Of Antioxidant Vitamins And Fatty Acids In Patients With Inactive Inflammatory Bowel Disease Clinical Nutrition 27, 4.
- ^ Roumeliotis, Stefanos; Dounousi, Evangelia; Bozikas, Andreas; Liakopoulos, Vassilios; Eleftheriadis, Theodoros (2019). Antioxidant Supplementation In Renal Replacement Therapy Patients: Is There Evidence? Oxidative Medicine And Cellular Longevity 2019, .
- ^ Jackson, P; Loughrey, C M; Lightbody, J H; McNamee, P T; Young, I S (1995). Effect Of Hemodialysis On Total Antioxidant Capacity And Serum Antioxidants In Patients With Chronic Renal Failure Clinical Chemistry 41, 8.
- ^ Deicher, Robert; Ziai, Farzad; Bieglmayer, Christian; Schillinger, Martin; Hörl, Walter H. (2005). Low Total Vitamin C Plasma Level Is A Risk Factor For Cardiovascular Morbidity And Mortality In Hemodialysis Patients Journal Of The American Society Of Nephrology 16, 6.
- ^ Hood, James; Canham, John E.; Sauberlich, Howerde E.; Baker, Eugene M.; Hodges, Robert E. (1971). Clinical Manifestations Of Ascorbic Acid Deficiency In Man The American Journal Of Clinical Nutrition 24, 4.
- ^ Maxfield L; Crane JS (2022). Vitamin C Deficiency. , .
- ^ Gan, Runye; Eintracht, Shaun; Hoffer, L. John (2008). Vitamin C Deficiency In A University Teaching Hospital Journal Of The American College Of Nutrition 27, 3.
- ^ Lykkesfeldt, Jens; Frei, Balz; Birlouez-Aragon, Ines (2012). Authors' Perspective: What Is The Optimum Intake Of Vitamin C In Humans? Critical Reviews In Food Science And Nutrition 52, 9.
- ^ a b Boffetta, Paolo; Greenwood, Darren C; Tonstad, Serena; Vatten, Lars J; Norat, Teresa; Riboli, Elio, et al. (2018). Dietary Intake And Blood Concentrations Of Antioxidants And The Risk Of Cardiovascular Disease, Total Cancer, And All-Cause Mortality: A Systematic Review And Dose-Response Meta-Analysis Of Prospective Studies The American Journal Of Clinical Nutrition 108, 5.
- ^ a b Levine, M; Conry-Cantilena, C; Wang, Y; Welch, R W; Washko, P W; Dhariwal, K R, et al. (1996). Vitamin C Pharmacokinetics In Healthy Volunteers: Evidence For A Recommended Dietary Allowance. Proceedings Of The National Academy Of Sciences 93, 8.
- ^ Malo, C.; Wilson, J. X. (2000). Glucose Modulates Vitamin C Transport In Adult Human Small Intestinal Brush Border Membrane Vesicles The Journal Of Nutrition 130, 1.
- ^ Lykkesfeldt, Jens; Tveden-Nyborg, Pernille (2019). The Pharmacokinetics Of Vitamin C Nutrients 11, 10.
- ^ a b c d e Padayatty, Sebastian J.; Sun, He; Wang, Yaohui; Riordan, Hugh D.; Hewitt, Stephen M.; Katz, Arie, et al. (2004). Vitamin C Pharmacokinetics: Implications For Oral And Intravenous Use Annals Of Internal Medicine 140, 7.
- ^ Hickey, Stephen; Roberts, Hilary J.; Miller, Nicholas J. (2008). Pharmacokinetics Of Oral Vitamin C Journal Of Nutritional & Environmental Medicine 17, 3.
- ^ Dałek, Paulina; Borowik, Tomasz; Foryś, Aleksander; Łukawski, Maciej; Langner, Marek; Witkiewicz, Wojciech, et al. (2019). New Oral Liposomal Vitamin C Formulation: Properties And Bioavailability Journal Of Liposome Research 30, 3.
- ^ Davis JL; Paris HL; Beals JW; Binns SE; Giordano GR; Scalzo RL, et al. (2016). Liposomal-encapsulated Ascorbic Acid: Influence on Vitamin C Bioavailability and Capacity to Protect Against Ischemia-Reperfusion Injury. Nutr Metab Insights 9, .
- ^ Hemilä, Harri (1999). Vitamin C Supplementation And Common Cold Symptoms: Factors Affecting The Magnitude Of The Benefit Medical Hypotheses 52, 2.
- ^ Bu, Huaien; Wang, Hongwu; Zhao, Ye; Tseng, Yiider; Ran, Li; Zhao, Wenli, et al. (2018). Extra Dose Of Vitamin C Based On A Daily Supplementation Shortens The Common Cold: A Meta-Analysis Of 9 Randomized Controlled Trials BioMed Research International 2018, .
- ^ a b c d Douglas RM; Hemila H; D'Souza R; Chalker EB; Treacy B (2004). Vitamin C for preventing and treating the common cold. Cochrane Database Syst Rev , 4.
- ^ Li, Yin; Li, Guoping (2016). Is Vitamin C Beneficial To Patients With CAP? Current Infectious Disease Reports 18, 8.
- ^ Gekara, Nelson; Erttmann, Saskia F. (2019). Hydrogen Peroxide Release By Bacteria Suppresses Inflammasome-Dependent Innate Immunity Nature Communications 10, 1.
- ^ a b Myint, Phyo K; Luben, Robert; Wilson, Andrew M.; Wareham, Nicholas J.; Khaw, Kay-Tee; Clark, Allan (2019). Plasma Vitamin C Concentrations And Risk Of Incident Respiratory Diseases And Mortality In The European Prospective Investigation Into Cancer-Norfolk Population-Based Cohort Study European Journal Of Clinical Nutrition 73, 11.
- ^ Chalker, Elizabeth; Hemilä, Harri (2019). Vitamin C Can Shorten The Length Of Stay In The ICU: A Meta-Analysis Nutrients 11, 4.
- ^ a b Rosario, Nelson; Bonini, Sergio; Anderson, Sandra D; Weiler, John M.; Randolph, Christopher; Craig, Timothy J., et al. (2010). Pathogenesis, Prevalence, Diagnosis, And Management Of Exercise-Induced Bronchoconstriction: A Practice Parameter Annals Of Allergy, Asthma & Immunology 105, 6.
- ^ Hemilä H (2013). Vitamin C may alleviate exercise-induced bronchoconstriction: a meta-analysis. BMJ Open 3, 6.
- ^ Merchant, Anwar T.; Curhan, Gary; Bendich, Adrianne; Singh, Vishwa N.; Willett, Walter C.; Fawzi, Wafaie W. (2004). Vitamin Intake Is Not Associated With Community-Acquired Pneumonia In U.S. Men The Journal Of Nutrition 134, 2.
- ^ Neuman, Mark I.; Willett, Walter C.; Curhan, Gary C. (2007). Vitamin And Micronutrient Intake And The Risk Of Community-Acquired Pneumonia In US Women The American Journal Of Medicine 120, 4.
- ^ Khan, Suleman; Hakak, Saqib; Deepa, N.; Prabadevi, B.; Dev, Kapal; Trelova, Silvia (2022). Detecting COVID-19-Related Fake News Using Feature Extraction Frontiers In Public Health 9, .
- ^ Hemilä H; Douglas RM (1999). Vitamin C and acute respiratory infections. Int J Tuberc Lung Dis 3, 9.
- ^ Glazebrook, A. J.; Thomson, Scott (1942). The Administration Of Vitamin C In A Large Institution And Its Effect On General Health And Resistance To Infection Journal Of Hygiene 42, 1.
- ^ Kimbarowski JA; Mokrow NJ (1967). [Colored precipitation reaction of the urine according to Kimbarowski (FARK) as an index of the effect of ascorbic acid during treatment of viral influenza]. Dtsch Gesundheitsw 22, 51.
- ^ Hunt C; Chakravorty NK; Annan G; Habibzadeh N; Schorah CJ (1994). The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. Int J Vitam Nutr Res 64, 3.
- ^ Anah CO; Jarike LN; Baig HA (1980). High dose ascorbic acid in Nigerian asthmatics. Trop Geogr Med 32, 2.
- ^ 10.1016/s0140-6736(94)91399-4
- ^ Fogarty, A.; Lewis, S. A.; Scrivener, S. L.; Antoniak, M.; Pacey, S.; Pringle, M., et al. (2003). Oral Magnesium And Vitamin C Supplements In Asthma: A Parallel Group Randomized Placebo-Controlled Trial Clinical & Experimental Allergy 33, 10.
- ^ Luo, Jie; Shen, Li; Zheng, Di (2014). Association Between Vitamin C Intake And Lung Cancer: A Dose-Response Meta-Analysis Scientific Reports 4, 1.
- ^ Kawamura, Takuji; Muraoka, Isao (2018). Exercise-Induced Oxidative Stress And The Effects Of Antioxidant Intake From A Physiological Viewpoint Antioxidants 7, 9.
- ^ a b Nieman, David C.; Wentz, Laurel M. (2019). The Compelling Link Between Physical Activity And The Body'S Defense System Journal Of Sport And Health Science 8, 3.
- ^ Walsh, Neil P; Oliver, Samuel J (2015). Exercise, Immune Function And Respiratory Infection: An Update On The Influence Of Training And Environmental Stress Immunology & Cell Biology 94, 2.
- ^ a b Thompson, Dylan; Williams, Clyde; McGregor, Stephen J.; Nicholas, Ceri W.; McArdle, Frank; Jackson, Malcolm J., et al. (2001). Prolonged Vitamin C Supplementation And Recovery From Demanding Exercise International Journal Of Sport Nutrition And Exercise Metabolism 11, 4.
- ^ Bryer, S.C.; Goldfarb, A.H. (2006). Effect Of High Dose Vitamin C Supplementation On Muscle Soreness, Damage, Function, And Oxidative Stress To Eccentric Exercise International Journal Of Sport Nutrition And Exercise Metabolism 16, 3.
- ^ 10.1016/0304-3959(92)90037-c
- ^ Thompson, Dylan; Hill, J.; Hurst, T.; Powell, J. R.; Williams, C.; Bailey, D. M. (2004). Prolonged Vitamin C Supplementation And Recovery From Eccentric Exercise Graefe's Archive For Clinical And Experimental Ophthalmology 92, 1-2.
- ^ Garcia-Roves, Pablo; Thompson, D.; Williams, C.; McGregor, S. J.; McArdle, F.; Jackson, M. J. (2003). Post-exercise Vitamin C Supplementation And Recovery From Demanding Exercise Graefe's Archive For Clinical And Experimental Ophthalmology 89, 3.
- ^ Wadley, Glenn; Lamon, Séverine; Mason, Shaun; Morrison, Dale; Russell, A.P; Gatta, Paul, et al. (2015). Vitamin C And E Supplementation Prevents Some Of The Cellular Adaptations To Endurance-Training In Humans Free Radical Biology And Medicine 89, .
- ^ a b Wiig, Håvard; Raastad, Truls; Benestad, Haakon Breien; Paur, Ingvild; Paulsen, Gøran; Cumming, Kristoffer T., et al. (2014). Vitamin C And E Supplementation Hampers Cellular Adaptation To Endurance Training In Humans: A Double-Blind, Randomised, Controlled Trial The Journal Of Physiology 592, 8.
- ^ 10.1249/jsr.0b013e31825e19cd
- ^ Paschalis, Vassilis; Theodorou, Anastasios A.; Kyparos, Antonios; Zafeiridis, Andreas; Panayiotou, George; Vrabas, Ioannis S., et al. (2014). Low Vitamin C Values Are Linked With Decreased Physical Performance And Increased Oxidative Stress: Reversal By Vitamin C Supplementation European Journal Of Nutrition 55, 1.
- ^ Dela, F.; Mikines, K. J.; Von Linstow, M.; Secher, N. H.; Galbo, H. (2006). Effect Of Training On Insulin-Mediated Glucose Uptake In Human Muscle American Journal Of Physiology-Endocrinology And Metabolism 263, 6.
- ^ Duarte, José Alberto; Powers, Scott K.; Kavazis, Andreas N.; Talbert, Erin E. (2009). Reactive Oxygen Species Are Signalling Molecules For Skeletal Muscle Adaptation Experimental Physiology 95, 1.
- ^ Stumvoll, Michael; Ristow, Michael; Birringer, Marc; Zarse, Kim; Oberbach, Andreas; Klöting, Nora, et al. (2009). Antioxidants Prevent Health-Promoting Effects Of Physical Exercise In Humans Proceedings Of The National Academy Of Sciences 106, 21.
- ^ Nielsen, Søren; Rose, Adam J; Akerstrom, Thorbjorn; Pedersen, Bente Klarlund; Fischer, Christian P; Lykkesfeldt, Jens, et al. (2011). Effect Of Antioxidant Supplementation On Insulin Sensitivity In Response To Endurance Exercise Training American Journal Of Physiology-Endocrinology And Metabolism 300, 5.
- ^ Peters, E. (1997). Exercise, Immunology And Upper Respiratory Tract Infections Endoscopy 18, S 1.
- ^ Nieman, David C.; Henson, Dru A.; Butterworth, Diane E.; Warren, Beverly J.; Davis, J. Mark; Fagoaga, Omar R., et al. (1997). Vitamin C Supplementation Does Not Alter The Immune Response To 2.5 Hours Of Running International Journal Of Sport Nutrition 7, 3.
- ^ Krause, R.; Patruta, S.; Daxböck, F.; Fladerer, P.; Biegelmayer, C.; Wenisch, C. (2001). Effect Of Vitamin C On Neutrophil Function After High-Intensity Exercise European Journal Of Clinical Investigation 31, 3.
- ^ Vinci, Debra M.; Opiela, Shannon J.; Morrow, Jason D.; Nieman, David C.; Henson, Dru A.; McAnulty, Steve R., et al. (2002). Influence Of Vitamin C Supplementation On Oxidative And Immune Changes After An Ultramarathon Journal Of Applied Physiology 92, 5.
- ^ Tauler, P.; Aguiló, A.; Fuentespina, E.; Tur, J.; Pons, A. (2002). Diet Supplementation With Vitamin E, Vitamin C And Β-Carotene Cocktail Enhances Basal Neutrophil Antioxidant Enzymes In Athletes Pflügers Archiv - European Journal Of Physiology 443, 5.
- ^ a b Robson, Paula J.; Bouic, Patrick J.D.; Myburgh, Kathryn H. (2003). Antioxidant Supplementation Enhances Neutrophil Oxidative Burst In Trained Runners Following Prolonged Exercise International Journal Of Sport Nutrition And Exercise Metabolism 13, 3.
- ^ Ferrer, M D; Sureda, Antoni; Mestre, Antonia; Tur, Josep A.; Pons, Antoni (2013). Prevention Of Neutrophil Protein Oxidation With Vitamins C And E Diet Supplementation Without Affecting The Adaptive Response To Exercise International Journal Of Sport Nutrition And Exercise Metabolism 23, 1.
- ^ Tauler, Pedro; Sureda, Antoni; Aguiló, Antoni; Cases, Nuria; Llompart, Isabel; Tur, Josep A., et al. (2008). Influence Of An Antioxidant Vitamin-Enriched Drink On Pre- And Post-Exercise Lymphocyte Antioxidant System Annals Of Nutrition And Metabolism 52, 3.
- ^ Moor de Burgos A; Wartanowicz M; Ziemlański S (1992). Blood vitamin and lipid levels in overweight and obese women. Eur J Clin Nutr 46, 11.
- ^ Canoy, Dexter; Wareham, Nicholas; Welch, Ailsa; Bingham, Sheila; Luben, Robert; Day, Nicholas, et al. (2005). Plasma Ascorbic Acid Concentrations And Fat Distribution In 19 068 British Men And Women In The European Prospective Investigation Into Cancer And Nutrition Norfolk Cohort Study The American Journal Of Clinical Nutrition 82, 6.
- ^ Park, Younghyun; Jang, Joonseong; Lee, Dongju; Yoon, Michung (2018). Vitamin C Inhibits Visceral Adipocyte Hypertrophy And Lowers Blood Glucose Levels In High-Fat-Diet-Induced Obese C57BL/6J Mice Biomedical Science Letters 24, 4.
- ^ Thorin, Eric; Zahradka, Peter; Taylor, Carla; Paquet, Eric; Le Couteur, David; Massip, Laurent, et al. (2009). Vitamin C Restores Healthy Aging In A Mouse Model For Werner Syndrome The FASEB Journal 24, 1.
- ^ Johnston, Carol S; Corte, Corinne; Swan, Pamela D (2006). Marginal Vitamin C Status Is Associated With Reduced Fat Oxidation During Submaximal Exercise In Young Adults Nutrition & Metabolism 3, 1.
- ^ 10.1016/s0166-2236(99)01543-x
- ^ Lykkesfeldt, Jens; Tveden-Nyborg, Pernille; Hansen, Stine (2014). Does Vitamin C Deficiency Affect Cognitive Development And Function? Nutrients 6, 9.
- ^ Harrison, Fiona; Dawes, S.M.; Meredith, M.E.; Babaev, V.R.; Li, L.; May, J.M. (2010). Low Vitamin C And Increased Oxidative Stress And Cell Death In Mice That Lack The Sodium-Dependent Vitamin C Transporter SVCT2 Free Radical Biology And Medicine 49, 5.
- ^ Sotiriou, Sotiria; Gispert, Suzana; Cheng, Jun; Wang, Yaohui; Chen, Amy; Hoogstraten-Miller, Shelley, et al. (2002). Ascorbic-acid Transporter Slc23a1 Is Essential For Vitamin C Transport Into The Brain And For Perinatal Survival Nature Medicine 8, 5.
- ^ Lykkesfeldt, Jens; Tveden-Nyborg, Pernille; Christen, Stephan; Vogt, Lucile; Schjoldager, Janne G.; Jeannet, Natalie, et al. (2012). Maternal Vitamin C Deficiency During Pregnancy Persistently Impairs Hippocampal Neurogenesis In Offspring Of Guinea Pigs Plos One 7, 10.
- ^ Lykkesfeldt, Jens; Tveden-Nyborg, Pernille; Johansen, Louise Kruse; Raida, Zindy; Villumsen, Charlotte Krogh; Larsen, Jytte Overgaard (2009). Vitamin C Deficiency In Early Postnatal Life Impairs Spatial Memory And Reduces The Number Of Hippocampal Neurons In Guinea Pigs The American Journal Of Clinical Nutrition 90, 3.
- ^ Adlard, B. P. F.; De Souza, S. W.; Moon, S. (1974). Ascorbic Acid In Fetal Human Brain Archives Of Disease In Childhood 49, 4.
- ^ Zalani, Sunita; Rajalakshmi, R.; Parekh, L. J. (1989). Ascorbic Acid Concentration Of Human Fetal Tissues In Relation To Fetal Size And Gestational Age British Journal Of Nutrition 61, 3.
- ^ Huang, Wen-Juan; Zhang, Xia; Chen, Wei-Wei (2016). Role Of Oxidative Stress In Alzheimer's Disease Biomedical Reports 4, 5.
- ^ Mecocci, Patrizia; Polidori, M. Cristina (2002). Plasma Susceptibility To Free Radical-Induced Antioxidant Consumption And Lipid Peroxidation Is Increased In Very Old Subjects With Alzheimer Disease Journal Of Alzheimer's Disease 4, 6.
- ^ Foy, C.J. (1999). Plasma Chain-Breaking Antioxidants In Alzheimer's Disease, Vascular Dementia And Parkinson's Disease QJM: An International Journal Of Medicine 92, 1.
- ^ Gruber AJ; Pope HG Jr; Brown ME (1996). Do patients use marijuana as an antidepressant? Depression 4, 2.
- ^ Morris, Martha Clare; Beckett, Laurel A.; Scherr, Paul A.; Hebert, Liesi E.; Bennett, David A.; Field, Terry S., et al. (1998). Vitamin E And Vitamin C Supplement Use And Risk Of Incident Alzheimer Disease Alzheimer Disease & Associated Disorders 12, 3.
- ^ Masaki, K. H.; Losonczy, K. G.; Izmirlian, G.; Foley, D. J.; Ross, G. W.; Petrovitch, H., et al. (2000). Association Of Vitamin E And C Supplement Use With Cognitive Function And Dementia In Elderly Men Neurology 54, 6.
- ^ Engelhart MJ; Geerlings MI; Ruitenberg A; van Swieten JC; Hofman A; Witteman JC, et al. (2002). Dietary intake of antioxidants and risk of Alzheimer disease. JAMA 287, 24.
- ^ Tveden-Nyborg, Pernille; Lykkesfeldt, Jens (2009). Does Vitamin C Deficiency Result In Impaired Brain Development In Infants? Redox Report 14, 1.
- ^ Eldridge, C F; Bunge, M B; Bunge, R P; Wood, P M (1987). Differentiation Of Axon-Related Schwann Cells In Vitro. I. Ascorbic Acid Regulates Basal Lamina Assembly And Myelin Formation. Journal Of Cell Biology 105, 2.
- ^ Lee, Ji-Yeon; Chang, Mi-Yoon; Park, Chang-Hwan; Kim, Hye-Young; Kim, Jin-Hyuk; Son, Hyeon, et al. (2003). Ascorbate-induced Differentiation Of Embryonic Cortical Precursors Into Neurons And Astrocytes Journal Of Neuroscience Research 73, 2.
- ^ Flashman, Emily; Davies, Sarah; Yeoh, Kar Kheng; Schofield, Christopher J (2010). Investigating The Dependence Of The Hypoxia-Inducible Factor Hydroxylases (Factor Inhibiting HIF And Prolyl Hydroxylase Domain 2) On Ascorbate And Other Reducing Agents Biochemical Journal 427, 1.
- ^ Tomita S; Ueno M; Sakamoto M; Kitahama Y; Ueki M; Maekawa N, et al. (2003). Defective brain development in mice lacking the Hif-1alpha gene in neural cells. Mol Cell Biol 23, 19.
- ^ Colunga Biancatelli, Ruben Manuel Luciano; Berrill, Max; Marik, Paul E. (2019). The Antiviral Properties Of Vitamin C Expert Review Of Anti-infective Therapy 18, 2.
- ^ Downing, C; Schorah, C J; Piripitsi, A; Gallivan, L; Al-Hazaa, A H; Sanderson, M J, et al. (1996). Total Vitamin C, Ascorbic Acid, And Dehydroascorbic Acid Concentrations In Plasma Of Critically Ill Patients The American Journal Of Clinical Nutrition 63, 5.
- ^ 10.1016/s0022-4804(02)00083-5
- ^ a b Marik, Paul E. (2018). Vitamin C For The Treatment Of Sepsis: The Scientific Rationale Pharmacology & Therapeutics 189, .
- ^ Natarajan, Ramesh; Fisher, Bernard J.; Kraskauskas, Donatas; Martin, Erika J.; Farkas, Daniela; Puri, Puneet, et al. (2013). Attenuation Of Sepsis-Induced Organ Injury In Mice By Vitamin C Journal Of Parenteral And Enteral Nutrition 38, 7.
- ^ Natarajan, Ramesh; Fisher, Bernard J.; Seropian, Ignacio M.; Kraskauskas, Donatas; Thakkar, Jay N.; Voelkel, Norbert F., et al. (2011). Ascorbic Acid Attenuates Lipopolysaccharide-Induced Acute Lung Injury* Critical Care Medicine 39, 6.
- ^ Liu, Yan-Cun; Zhai, Jian-Hua; Lu, Bin; Qi, Hai-Xia; Yao, Ying; Shou, Song-Tao, et al. (2017). The Parenteral Vitamin C Improves Sepsis And Sepsis-Induced Multiple Organ Dysfunction Syndrome Via Preventing Cellular Immunosuppression Mediators Of Inflammation 2017, .
- ^ Kramer, David; Ojielo, Charles; Damm, Tessa; Cassity, Evan; Wieliczko, Aleksandra; Halquist, Matthew, et al. (2019). Effect Of Vitamin C Infusion On Organ Failure And Biomarkers Of Inflammation And Vascular Injury In Patients With Sepsis And Severe Acute Respiratory Failure Jama 322, 13.
- ^ Marik, Paul E.; Khangoora, Vikramjit; Rivera, Racquel; Hooper, Michael H.; Catravas, John (2017). Hydrocortisone, Vitamin C, And Thiamine For The Treatment Of Severe Sepsis And Septic Shock Chest 151, 6.
- ^ Cooper LT Jr (2009). Myocarditis. N Engl J Med 360, 15.
- ^ Bu, Huaien; Wang, Hongwu; Zhao, Ye; Tseng, Yiider; Chen, Shuangdi; Zhao, Wenli, et al. (2019). Clinical Effect Of Intravenous Vitamin C On Viral Myocarditis In Children: A Systematic Review And Meta-Analysis Evidence-Based Complementary And Alternative Medicine 2019, .
- ^ a b Chu, Chin-Chen; Chen, Jen-Yin; Chang, Chia-Yu; Feng, Ping-Hsun; So, Edmund Cheng; Hu, Miao-Lin (2009). Plasma Vitamin C Is Lower In Postherpetic Neuralgia Patients And Administration Of Vitamin C Reduces Spontaneous Pain But Not Brush-evoked Pain The Clinical Journal Of Pain 25, 7.
- ^ Schencking M; Sandholzer H; Frese T (2010). Intravenous administration of vitamin C in the treatment of herpetic neuralgia: two case reports. Med Sci Monit 16, 5.
- ^ Chen JY; Chu CC; So EC; Hsing CH; Hu ML (2006). Treatment of postherpetic neuralgia with intravenous administration of vitamin C. Anesth Analg 103, 6.
- ^ Mikirova N; Hunninghake R (2014). Effect of high dose vitamin C on Epstein-Barr viral infection. Med Sci Monit 20, .
- ^ a b Cheng LL; Liu YY; Li B; Li SY; Ran PX (2012). [An in vitro study on the pharmacological ascorbate treatment of influenza virus]. Zhonghua Jie He He Hu Xi Za Zhi 35, 7.
- ^ Furuya A; Uozaki M; Yamasaki H; Arakawa T; Arita M; Koyama AH (2008). Antiviral effects of ascorbic and dehydroascorbic acids in vitro. Int J Mol Med 22, 4.
- ^ a b c Skidmore, Becky; Cooley, Kieran; Fritz, Heidi; Flower, Gillian; Weeks, Laura; Callachan, Michael, et al. (2014). Intravenous Vitamin C And Cancer Integrative Cancer Therapies 13, 4.
- ^ Cameron, Ewan; Pauling, Linus (1978). Supplemental Ascorbate In The Supportive Treatment Of Cancer: Reevaluation Of Prolongation Of Survival Times In Terminal Human Cancer Proceedings Of The National Academy Of Sciences 75, 9.
- ^ Wall, Clare; Wilson, Michelle K; Baguley, Bruce C; Jameson, Michael B; Findlay, Michael P (2014). Review Of High-Dose Intravenous Vitamin C As An Anticancer Agent Asia-Pacific Journal Of Clinical Oncology 10, 1.
- ^ Jacobs, Carmel; Ng, Terry; Hutton, Brian; Shorr, Risa; Clemons, Mark (2015). Is There A Role For Oral Or Intravenous Ascorbate (Vitamin C) In Treating Patients With Cancer? A Systematic Review The Oncologist 20, 2.
- ^ Furqan, Muhammad; Schoenfeld, Joshua D; Sandhu, Sonia; Buettner, Garry R; Spitz, Douglas R; Cullen, J J, et al. (2017). O 2 ⋅− And H 2 O 2 -Mediated Disruption Of Fe Metabolism Causes The Differential Susceptibility Of NSCLC And GBM Cancer Cells To Pharmacological Ascorbate Cancer Cell 31, 4.
- ^ 10.3390/antiox7090115
- ^ a b c Buettner, Garry R; Cullen, J J; Berg, Dale; Halfdanarson, Thorvardur Ragnar; Bodeker, Kellie; Welsh, J. L., et al. (2013). Pharmacological Ascorbate With Gemcitabine For The Control Of Metastatic And Node-Positive Pancreatic Cancer (PACMAN): Results From A Phase I Clinical Trial Cancer Chemotherapy And Pharmacology 71, 3.
- ^ Burris, H A; Moore, M J; Andersen, J; Green, M R; Rothenberg, M L; Modiano, M R, et al. (1997). Improvements In Survival And Clinical Benefit With Gemcitabine As First-Line Therapy For Patients With Advanced Pancreas Cancer: A Randomized Trial. Journal Of Clinical Oncology Journal Of Clinical Oncology 15, 6.
- ^ a b Ma, Yan; Chapman, Julia; Levine, Mark; Polireddy, Kishore; Drisko, Jeanne; Chen, Qi (2014). High-Dose Parenteral Ascorbate Enhanced Chemosensitivity Of Ovarian Cancer And Reduced Toxicity Of Chemotherapy Science Translational Medicine 6, 222.
- ^ Creagan, Edward T.; Moertel, Charles G.; O'Fallon, Judith R.; Schutt, Allan J.; O'Connell, Michael J.; Rubin, Joseph, et al. (1979). Failure Of High-Dose Vitamin C (Ascorbic Acid) Therapy To Benefit Patients With Advanced Cancer New England Journal Of Medicine 301, 13.
- ^ Moertel, Charles G.; Fleming, Thomas R.; Creagan, Edward T.; Rubin, Joseph; O'Connell, Michael J.; Ames, Matthew M. (1985). High-Dose Vitamin C Versus Placebo In The Treatment Of Patients With Advanced Cancer Who Have Had No Prior Chemotherapy New England Journal Of Medicine 312, 3.
- ^ Yeom, Chang Hwan; Jung, Gyou Chul; Song, Keun Jeong (2007). Changes Of Terminal Cancer Patients' Health-related Quality Of Life After High Dose Vitamin C Administration Journal Of Korean Medical Science 22, 1.
- ^ Vollbracht C; Schneider B; Leendert V; Weiss G; Auerbach L; Beuth J (2011). Intravenous vitamin C administration improves quality of life in breast cancer patients during chemo-/radiotherapy and aftercare: results of a retrospective, multicentre, epidemiological cohort study in Germany. In Vivo 25, 6.
- ^ a b Stephenson, Christopher M.; Levin, Robert D.; Spector, Thomas; Lis, Christopher G. (2013). Phase I Clinical Trial To Evaluate The Safety, Tolerability, And Pharmacokinetics Of High-Dose Intravenous Ascorbic Acid In Patients With Advanced Cancer Cancer Chemotherapy And Pharmacology 72, 1.
- ^ Hoffer, L. John; Robitaille, Line; Zakarian, Robert; Melnychuk, David; Kavan, Petr; Agulnik, Jason, et al. (2015). High-Dose Intravenous Vitamin C Combined With Cytotoxic Chemotherapy In Patients With Advanced Cancer: A Phase I-II Clinical Trial Plos One 10, 4.
- ^ Inhorn, M. C.; Patrizio, P. (2015). Infertility Around The Globe: New Thinking On Gender, Reproductive Technologies And Global Movements In The 21St Century Human Reproduction Update 21, 4.
- ^ Mascarenhas, Maya N.; Flaxman, Seth R.; Boerma, Ties; Vanderpoel, Sheryl; Stevens, Gretchen A. (2012). National, Regional, And Global Trends In Infertility Prevalence Since 1990: A Systematic Analysis Of 277 Health Surveys PLOS Medicine 9, 12.
- ^ a b Zini, A.; Lamirande, E.; Gagnon, C. (1993). Reactive Oxygen Species In Semen Of Infertile Patients: Levels Of Superoxide Dismutase- And Catalase-Like Activities In Seminal Plasma And Spermatozoa International Journal Of Andrology 16, 3.
- ^ 10.1016/s0015-0282(16)57017-4
- ^ Akmal, Mohammed; Qadri, J.Q.; Al-Waili, Noori S.; Thangal, Shahiya; Haq, Afrozul; Saloom, Khelod Y. (2006). Improvement In Human Semen Quality After Oral Supplementation Of Vitamin C Journal Of Medicinal Food 9, 3.
- ^ Dawson EB; Harris WA; Teter MC; Powell LC (1992). Effect of ascorbic acid supplementation on the sperm quality of smokers. Fertil Steril 58, 5.
- ^ Ness AR; Khaw KT; Bingham S; Day NE (1996). Vitamin C status and blood pressure. J Hypertens 14, 4.
- ^ Juraschek, Stephen; Guallar, Eliseo; Appel, Lawrence J; Miller, Edgar R (2012). Effects Of Vitamin C Supplementation On Blood Pressure: A Meta-Analysis Of Randomized Controlled Trials The American Journal Of Clinical Nutrition 95, 5.
- ^ Ried K; Travica N; Sali A (2016). The acute effect of high-dose intravenous vitamin C and other nutrients on blood pressure: a cohort study. Blood Press Monit 21, 3.
- ^ Elbers, Paul; Spoelstra-de Man, Angelique M. E.; Oudemans-van Straaten, Heleen M. (2018). Making Sense Of Early High-Dose Intravenous Vitamin C In Ischemia/Reperfusion Injury Critical Care 22, 1.
- ^ Basili, Stefania; Violi, Francesco; Pignatelli, Pasquale; Raparelli, Valeria; Tanzilli, Gaetano; Mangieri, Enrico, et al. (2010). Intravenous Ascorbic Acid Infusion Improves Myocardial Perfusion Grade During Elective Percutaneous Coronary Intervention JACC: Cardiovascular Interventions 3, 2.
- ^ Wang, Zhi Jian; Hu, Wen Kun; Liu, Yu Yang; Shi, Dong Mei; Cheng, Wan Jun; Guo, Yong He, et al. (2014). The Effect Of Intravenous Vitamin C Infusion On Periprocedural Myocardial Injury For Patients Undergoing Elective Percutaneous Coronary Intervention Canadian Journal Of Cardiology 30, 1.
- ^ 10.1016/s1734-1140(10)70334-0
- ^ Block, Gladys; Jensen, Christopher D.; Dalvi, Tapashi B.; Norkus, Edward P.; Hudes, Mark; Crawford, Patricia B., et al. (2009). Vitamin C Treatment Reduces Elevated C-reactive Protein Free Radical Biology And Medicine 46, 1.
- ^ Ridker, Paul M.; Rifai, Nader; Pfeffer, Marc A.; Sacks, Frank; Braunwald, Eugene (1999). Long-Term Effects Of Pravastatin On Plasma Concentration Of C-reactive Protein Circulation 100, 3.
- ^ 10.1056/nejm200106283442601
- ^ Albert, Michelle A.; Danielson, Ellie; Rifai, Nader; Ridker, Paul M; For The Prince Investigators, (2001). Effect Of Statin Therapy On C-Reactive Protein Levels Jama 286, 1.
- ^ 10.1016/s0140-6736(10)60826-4
- ^ Mikirova, N.; Rogers, A.; Casciari, J.; Taylor, P. (2012). Effect Of High Dose Intravenous Ascorbic Acid On The Level Of Inflammation In Patients With Rheumatoid Arthritis Modern Research In Inflammation 01, 02.
- ^ Washko P; Rotrosen D; Levine M (1989). Ascorbic acid transport and accumulation in human neutrophils. J Biol Chem 264, 32.
- ^ Bergsten P; Amitai G; Kehrl J; Dhariwal KR; Klein HG; Levine M (1990). Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J Biol Chem 265, 5.
- ^ Pavlovic, Voja; Sarac, M. (2010). A Short Overview Of Vitamin C And Selected Cells Of The Immune System Open Medicine 6, 1.
- ^ Goldschmidt, M C (1991). Reduced Bactericidal Activity In Neutrophils From Scorbutic Animals And The Effect Of Ascorbic Acid On These Target Bacteria In Vivo And In Vitro The American Journal Of Clinical Nutrition 54, 6.
- ^ Goldschmidt MC; Masin WJ; Brown LR; Wyde PR (1988). The effect of ascorbic acid deficiency on leukocyte phagocytosis and killing of actinomyces viscosus. Int J Vitam Nutr Res 58, 3.
- ^ Bozonet, Stephanie; Carr, Anitra; Pullar, Juliet; Vissers, Margreet (2015). Enhanced Human Neutrophil Vitamin C Status, Chemotaxis And Oxidant Generation Following Dietary Supplementation With Vitamin C-Rich SunGold Kiwifruit Nutrients 7, 4.
- ^ de la Fuente M; Ferrández MD; Burgos MS; Soler A; Prieto A; Miquel J (1998). Immune function in aged women is improved by ingestion of vitamins C and E. Can J Physiol Pharmacol 76, 4.
- ^ Wu, Yangzhe; Yin, Zhinan; Kabelitz, Dieter; Kouakanou, Léonce; Xu, Yan; Peters, Christian, et al. (2019). Vitamin C Promotes The Proliferation And Effector Functions Of Human Γδ T Cells Cellular & Molecular Immunology 17, 5.
- ^ Jeong, Young-Joo; Hong, Seung-Woo; Kim, Jin-Hee; Jin, Dong-Hoon; Kang, Jae Seung; Lee, Wang Jae, et al. (2011). Vitamin C-treated Murine Bone Marrow-Derived Dendritic Cells Preferentially Drive Naïve T Cells Into Th1 Cells By Increased IL-12 Secretions Cellular Immunology 266, 2.
- ^ Manning, Jared; Mitchell, Birgitta; Appadurai, Daniel A.; Pierce, Laura Jean; Wang, Hongfang; Nganga, Vincent, et al. (2013). Vitamin C Promotes Maturation Of T-Cells Antioxidants & Redox Signaling 19, 17.
- ^ Briedé, Jacob; Huijskens, Mirelle J. A. J.; Walczak, Mateusz; Koller, Nicole; Senden-Gijsbers, Birgit L. M. G.; Schnijderberg, Melanie C., et al. (2014). Technical Advance: Ascorbic Acid Induces Development Of Double-Positive T Cells From Human Hematopoietic Stem Cells In The Absence Of Stromal Cells Journal Of Leukocyte Biology 96, 6.
- ^ Campbell, Jeff D.; Cole, Michael; Bunditrutavorn, Bongkot; Vella, Anthony T. (1999). Ascorbic Acid Is A Potent Inhibitor Of Various Forms Of T Cell Apoptosis Cellular Immunology 194, 1.
- ^ a b c Kim, Yejin; Kim, Hyemin; Bae, Seyeon; Choi, Jiwon; Lim, Sun Young; Lee, Naeun, et al. (2013). Vitamin C Is An Essential Factor On The Anti-viral Immune Responses Through The Production Of Interferon-α/β At The Initial Stage Of Influenza A Virus (H3N2) Infection Immune Network 13, 2.
- ^ Natarajan, Ramesh; Mohammed, Bassem; Fisher, Bernard; Kraskauskas, Donatas; Farkas, Daniela; Brophy, Donald, et al. (2013). Vitamin C: A Novel Regulator Of Neutrophil Extracellular Trap Formation Nutrients 5, 8.
- ^ Adekola K; Rosen ST; Shanmugam M (2012). Glucose transporters in cancer metabolism. Curr Opin Oncol 24, 6.
- ^ Mackenzie, Bryan; Hediger, Matthias A; Tsukaguchi, Hiroyasu; Tokui, Taro; Berger, Urs V.; Chen, Xing-Zhen, et al. (1999). A Family Of Mammalian Na+-dependent L-ascorbic Acid Transporters Nature 399, 6731.
- ^ Van Schaftingen, Emile; Linster, Carole L (2006). Vitamin C The FEBS Journal 274, 1.
- ^ Burant, Charles F; Rumsey, Steven C.; Kwon, Oran; Xu, Guo Wei; Simpson, Ian; Levine, Mark (1997). Glucose Transporter Isoforms GLUT1 And GLUT3 Transport Dehydroascorbic Acid Journal Of Biological Chemistry 272, 30.
- ^ Vera, Juan Carlos; Rivas, Coralia I.; Fischbarg, Jorge; Golde, David W. (1993). Mammalian Facilitative Hexose Transporters Mediate The Transport Of Dehydroascorbic Acid Nature 364, 6432.
- ^ Cantley, Lewis C; Roper, Jatin; Rivera, Keith; Yun, J.; Mullarky, E.; Lu, C., et al. (2015). Vitamin C Selectively Kills KRAS And BRAF Mutant Colorectal Cancer Cells By Targeting GAPDH Science 350, 6266.
- ^ Padayatty, Sj; Levine, M (2016). Vitamin C: The Known And The Unknown And Goldilocks Oral Diseases 22, 6.
- ^ Levine, Mark; Padayatty, Sebastian J.; Espey, Michael Graham (2011). Vitamin C: A Concentration-Function Approach Yields Pharmacology And Therapeutic Discoveries Advances In Nutrition 2, 2.
- ^ Buettner, Garry R; Cullen, J J; Doskey, Claire M.; Buranasudja, Visarut; Wagner, Brett A.; Wilkes, Justin G., et al. (2016). Tumor Cells Have Decreased Ability To Metabolize H2O2: Implications For Pharmacological Ascorbate In Cancer Therapy Redox Biology 10, .
- ^ Buettner, Garry R; Corpe, Christopher; Chen, Qi; Espey, Michael Graham; Krishna, Murali C.; Mitchell, James B., et al. (2005). Pharmacologic Ascorbic Acid Concentrations Selectively Kill Cancer Cells: Action As A Pro-Drug To Deliver Hydrogen Peroxide To Tissues Proceedings Of The National Academy Of Sciences 102, 38.
- ^ Chen, Qi; Espey, Michael Graham; Sun, Andrew Y.; Pooput, Chaya; Kirk, Kenneth L.; Krishna, Murali C., et al. (2008). Pharmacologic Doses Of Ascorbate Act As A Prooxidant And Decrease Growth Of Aggressive Tumor Xenografts In Mice Proceedings Of The National Academy Of Sciences 105, 32.
- ^ Furqan, Muhammad; Schoenfeld, Joshua D; Sandhu, Sonia; Buettner, Garry R; Spitz, Douglas R; Cullen, J J, et al. (2017). O2⋅− And H2O2-Mediated Disruption Of Fe Metabolism Causes The Differential Susceptibility Of NSCLC And GBM Cancer Cells To Pharmacological Ascorbate Cancer Cell 32, 2.
- ^ Oberley TD; Oberley LW (1997). Antioxidant enzyme levels in cancer. Histol Histopathol 12, 2.
- ^ Böhles, H; Schremmer, D; Zoller, W G; Biesalski, Hk; Mühlhöfer, A; Mrosek, S, et al. (2004). High-dose Intravenous Vitamin C Is Not Associated With An Increase Of Pro-Oxidative Biomarkers European Journal Of Clinical Nutrition 58, 8.
- ^ Harakeh, S; Jariwalla, R J; Pauling, L (1990). Suppression Of Human Immunodeficiency Virus Replication By Ascorbate In Chronically And Acutely Infected Cells. Proceedings Of The National Academy Of Sciences 87, 18.
- ^ Bendich, A; Langseth, L (1995). The Health Effects Of Vitamin C Supplementation: A Review. Journal Of The American College Of Nutrition 14, 2.
- ^ Rivers, Jerry M. (1987). Safety Of High-level Vitamin C Ingestion Annals Of The New York Academy Of Sciences 498, 1 Third Confere.
- ^ Tavill, A (2001). Diagnosis And Management Of Hemochromatosis Hepatology 33, 5.
- ^ Newberg, Andrew; Monti, Daniel A.; Mitchell, Edith; Bazzan, Anthony J.; Littman, Susan; Zabrecky, George, et al. (2012). Phase I Evaluation Of Intravenous Ascorbic Acid In Combination With Gemcitabine And Erlotinib In Patients With Metastatic Pancreatic Cancer Plos One 7, 1.
- ^ a b Riordan HD; Casciari JJ; González MJ; Riordan NH; Miranda-Massari JR; Taylor P, et al. (2005). A pilot clinical study of continuous intravenous ascorbate in terminal cancer patients. P R Health Sci J 24, 4.
- ^ Padayatty, Sebastian J.; Sun, Andrew Y.; Chen, Qi; Espey, Michael Graham; Drisko, Jeanne; Levine, Mark (2010). Vitamin C: Intravenous Use By Complementary And Alternative Medicine Practitioners And Adverse Effects Plos One 5, 7.
- ^ Campbell, G. Douglas (1975). Ascorbic Acid-Induced Hemolysis In G-6-PD Deficiency Annals Of Internal Medicine 82, 6.
- ^ Richards, J D; Rees, D C; Kelsey, H (1993). Acute Haemolysis Induced By High Dose Ascorbic Acid In Glucose-6-Phosphate Dehydrogenase Deficiency. Bmj 306, 6881.
- ^ Ibrahim IH; Sallam SM; Omar H; Rizk M (2006). Oxidative hemolysis of erythrocytes induced by various vitamins. Int J Biomed Sci 2, 3.
- ^ Rino, Pedro Bonifacio; Scolnik, Dennis; Fustiñana, Ana; Mitelpunkt, Alexis; Glatstein, Miguel (2014). Ascorbic Acid For The Treatment Of Methemoglobinemia American Journal Of Therapeutics 21, 4.
- ^ Rehman, Abdul; Shehadeh, Mohanad; Khirfan, Diala; Jones, Akhnuwhkh (2018). Severe Acute Haemolytic Anaemia Associated With Severe Methaemoglobinaemia In A G6PD-deficient Man BMJ Case Reports , .
- ^ Marik PE (2019). Is intravenous vitamin C contraindicated in patients with G6PD deficiency? Crit Care 23, 1.
- ^ Riordan HD; Riordan NH; Jackson JA; Casciari JJ; Hunninghake R; González MJ, et al. (2004). Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P R Health Sci J 23, 2.
- ^ Mashour, S.; Turner, J.F.; Merrell, R. (2000). Acute Renal Failure, Oxalosis, And Vitamin C Supplementation Chest 118, 2.
- ^ Holmes, R P; Assimos, Dean G. (2004). The Impact Of Dietary Oxalate On Kidney Stone Formation Urological Research 32, 5.
- ^ Lawton JM; Conway LT; Crosson JT; Smith CL; Abraham PA (1985). Acute oxalate nephropathy after massive ascorbic acid administration. Arch Intern Med 145, 5.
- ^ Wong, K.; Thomson, C.; Bailey, R. R.; McDIARMID, S.; Gardner, J. (1994). Acute Oxalate Nephropathy After A Massive Intravenous Dose Of Vitamin C Australian And New Zealand Journal Of Medicine 24, 4.
- ^ Buehner M; Pamplin J; Studer L; Hughes RL; King BT; Graybill JC, et al. (2016). Oxalate Nephropathy After Continuous Infusion of High-Dose Vitamin C as an Adjunct to Burn Resuscitation. J Burn Care Res 37, 4.
- ^ Gambaro, Giovanni; Ferraro, Pietro Manuel; Curhan, Gary C.; Taylor, Eric N. (2016). Total, Dietary, And Supplemental Vitamin C Intake And Risk Of Incident Kidney Stones American Journal Of Kidney Diseases 67, 3.
- ^ Taylor, E. N. (2004). Dietary Factors And The Risk Of Incident Kidney Stones In Men: New Insights After 14 Years Of Follow-up Journal Of The American Society Of Nephrology 15, 12.
- ^ Thomas, Laura D. K.; Elinder, Carl-Gustaf; Tiselius, Hans-Göran; Wolk, Alicja; Åkesson, Agneta (2013). Ascorbic Acid Supplements And Kidney Stone Incidence Among Men: A Prospective Study JAMA Internal Medicine 173, 5.
- ^ Robitaille, Line; Mamer, Orval A.; Miller, Wilson H.; Levine, Mark; Assouline, Sarit; Melnychuk, David, et al. (2009). Oxalic Acid Excretion After Intravenous Ascorbic Acid Administration Metabolism 58, 2.
Topics related to Nutrition
-
Exercise and Weight Loss
Exercise is promoted widely as a strategy for weight loss as it increases total daily energy expenditure and creates an energy deficit.
-
Liquid biopsy (cancer screening)
Liquid biopsies, a non-invasive cancer screening technology that includes GRAIL's Galleri test, could transform early detection and treatment by analyzing tumor-derived cells and components.
-
Zinc
Zinc is an essential nutrient that influences growth and development and plays critical roles in immune function, protein synthesis, wound healing, DNA synthesis, and cell division.
-
Hydrolyzed collagen
Hydrolyzed collagen, a mixture of peptides derived from collagen, may improve skin aging, decrease arthritis-induced pain, increase bone mineral density, and reduce hypertension.
-
Aerobic exercise
Aerobic exercise, physical activity that increases breathing and heart rate, promotes cardiovascular, brain, and whole-body health.
-
Exercise Intensity
Vigorous exercise exerts several benefits on cardiovascular health, metabolic health, and longevity.