Telomeres: controlling aging or just a biomarker? | Elissa Epel
Get the full length version of this episode as a podcast.
This episode will make a great companion for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
A cell's finite capacity to divide – a phenomenon known as replicative senescence – is an intrinsic feature of the body's cells and fundamental to the aging process. Telomeres in most human cells shorten during aging, suggesting that it is both a cause and a biomarker of aging. In this clip, Dr. Elissa Epel explains how new research using Mendelian randomization techniques is providing insights into the dual nature of telomeres.
Rhonda: In your experience, how much would you say that telomere length...so, you know, the telomeres get shorter with time and shorter telomeres are supposed to correspond to aging. How much would you say that telomere length regulates the aging process, like actually plays an active role, versus just is a biomarker, something that is just biomarking the aging process?
Elissa: That's a good question. So telomeres are one specific pathway of how a cell ages, and how our tissue ages. And the pathway is this, it's called replicative senescence and it's basically how long can that cell continue to divide, and divide, and replenish into new fresh young cells. So the telomeres, when they get too short, prevent that particular cell, whether it's an immune cell or a neuron in our hippocampus or the lining of our cardiovascular system, we need those cells to replenish throughout the decades. When it to the telomeres gets too short that cell stops dividing. And so it's basically a little window into how long can these cells continue dividing. If the telomeres are long, they have a long potential for replenishing tissue.
Elissa: One last point about genetics. So you were you earlier asked, like, is this a marker of aging or is it a mechanism? So it is probably both. And the way we know that it's the mechanism as well is... I mean, partly the example to some of these genetic disorders, but even more so, now we know that if you have a genetic propensity for long telomeres, it directly predicts less heart disease and dementia. So that was those kinds of Mendelian randomization studies are one of the best ways that we can say there's a direct physiological connection here.
Rhonda: Right yeah, I didn't know there were any Mendelian randomization studies on it. That is very interesting. So cardiovascular disease and dementia are two one's health outcomes that seem to be effective.
Elissa: Right. And then as I said the higher cancer risk for some of these.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A general term referring to cognitive decline that interferes with normal daily living. Dementia commonly occurs in older age and is characterized by progressive loss of memory, executive function, and reasoning. Approximately 70 percent of all dementia cases are due to Alzheimer’s disease.
A small organ located within the brain's medial temporal lobe. The hippocampus is associated primarily with memory (in particular, the consolidation of short-term memories to long-term memories), learning, and spatial navigation. Amyloid-beta plaque accumulation, tau tangle formation, and subsequent atrophy in the hippocampus are early indicators of Alzheimer’s disease.
A research method that provides evidence of links between modifiable risk factors and disease based on genetic variants within a population. Mendelian randomization studies are less likely to be affected by confounding or reverse causation than other types of studies, but since MR is based on assumptions, the likelihood of the assumptions must be taken into consideration.
Senescence is a response to stress in which damaged cells suspend normal growth and metabolism. While senescence is vital for embryonic development, wound healing, and cancer immunity, accumulation of senescent cells causes increases inflammation and participates in the phenotype of aging.
Distinctive structures comprised of short, repetitive sequences of DNA located on the ends of chromosomes. Telomeres form a protective “cap” – a sort of disposable buffer that gradually shortens with age – that prevents chromosomes from losing genes or sticking to other chromosomes during cell division. When the telomeres on a cell’s chromosomes get too short, the chromosome reaches a “critical length,” and the cell stops dividing (senescence) or dies (apoptosis). Telomeres are replenished by the enzyme telomerase, a reverse transcriptase.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Get email updates with the latest curated healthspan research
Support our work
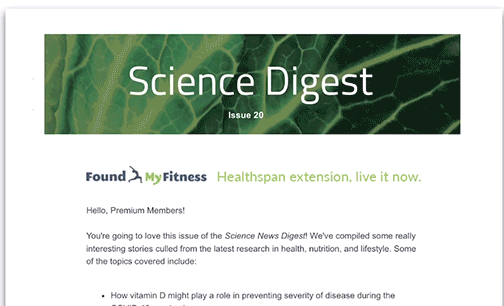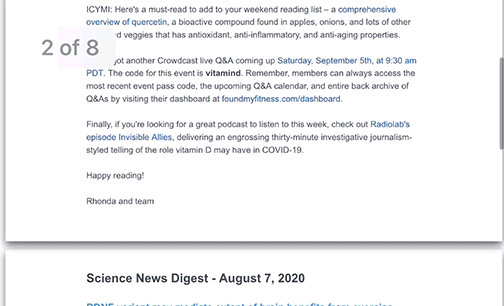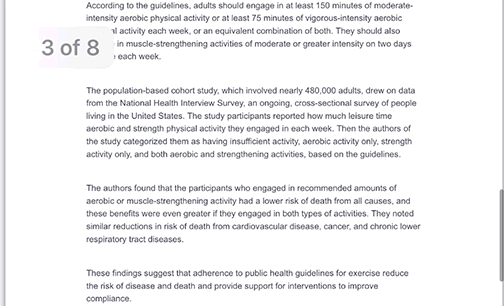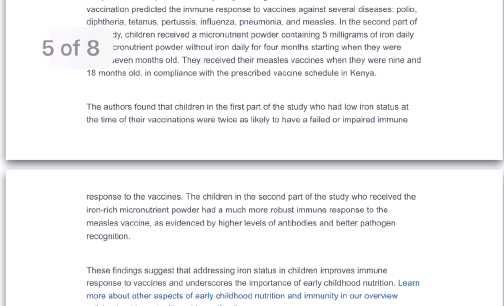
Every other week premium members receive a special edition newsletter that summarizes all of the latest healthspan research.
Telomeres News
- Lifelong competitive sport linked to slower biological aging through reduced cellular stress.
- Hyperbaric oxygen therapy reverses aspects of the cellular aging process in older adults, boosting immune cell function.
- Hyperbaric oxygen trial discovered a lengthening of up to 38% of the telomeres, decrease of up to 37% in senescent cells in certain cell populations
- Removing senescent cardiac muscle cells from the hearts of aged mice, both genetically and using drugs, reduces hypertrophy and fibrosis in aged heart
- Aerobic exercise (120 minutes per week) significantly lengthened telomeres, a biomarker for healthy aging, in white blood cells after 24 weeks.






