#70 Dr. Eran Elinav: Microbiome Insights into Personalized Response to Diet, Obesity, and Leaky Gut
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
Eran Elinav, MD, PhD, is a professor of immunology and principal investigator at the Weizmann Institute of Science in Tel Aviv, Israel, where he co-directs the Personalized Nutrition Project. Dr. Elinav is also a principal investigator at the German Cancer Research Center in Heidelberg, Germany. His research focuses on understanding the complex interactions between humans and the bacteria that reside in their gut and how these interactions shape human health and disease.
In this episode, Dr. Elinav and I discuss:
-
The circadian rhythmicity of the microbiome
-
The differing roles of genes versus the environment in determining the make-up of the microbiome
-
The influence of the microbiome on cholesterol and triglyceride levels
-
Effect of artificial sweeteners on the microbiome
-
Microbiome-related dynamics of weight regain and why some people have a harder time keeping weight off
-
Leaky gut and the role of metabolic endotoxemia in disease
-
Interindividual differences in response to probiotics
-
Whether advances in bacteriophage therapy will solve antibiotic resistance
-
Bacterial role in TMAO risk
-
Smoking cessation-associated weight gain
Expanding our conception of the human to include our microbes — the human holobiont
"The holobiont concept, in which you can regard a human as a combined set of microbes and human cells, could contribute to many of the more complex health outcomes that are so concerning to many of us."- Eran Elinav, MD, PhD Click To Tweet
More than a century ago, immunologist Élie Metchnikoff theorized that the secret to robust health and long life lay within the gut microbiome – the collective genomes of the trillions of microorganisms that reside in the human intestine. The popularity and acceptance of Metchnikoff's theories waned over time but in recent decades have seen a revival, as researchers, healthcare professionals, and the lay public alike have come to appreciate that these microorganisms, often referred to as commensals (a Latin term that means "eating at the same table"), play seemingly countless roles in human physiology, influencing nearly every aspect of our physical, emotional, and psychological well-being. In essence, our microbiome defines us.
Dr. Elinav explains that the factors that shape and alter a person's microbiome exert their influence throughout the lifespan, starting at birth (and even before), governed by genetic drivers and early environmental exposures. The genetic influence on the microbiome is miniscule, however, accounting for less than 2 percent of its overall makeup and character. The lion's share is relegated to environmental exposures, the first of which occurs at birth, when a host of microbes bombard the previously sterile infant.
Variations in these exposures, starting with delivery mode – vaginal versus Caesarean – and continuing for about the first three years of life, potentially expose an infant to vastly different microbial populations, influencing nearly every aspect of their health. For example, growing up in an overly hygienic environment increases a child's risk for developing autoimmune diseases, but exposure to dirt, especially from soil, pets, and roaches, in the first year of life may provide protection against asthma and allergy-related skin disease. And a growing body of evidence hints that early life antibiotic use predisposes a person to obesity and other chronic diseases later in life. Similarly, the waning gut microbial populations and subsequent loss of protectiveness in old age may arise from age-related changes in the gut and environmental exposures such as increased medication use. And even cigarette smoking can alter the gut microbiome in such a way that drives weight gain when smokers quit.
But the greatest influence on the types and numbers of microbes that populate our gut microbiome throughout the lifespan comes from what – and surprisingly, when – we eat, says Dr. Elinav. Indeed, dietary components and microbial circadian rhythmicity call the shots on who stays and who goes in the gut.
What we eat matters.
"Of all the different environmental factors that affect us humans – our stress levels, the medications we take, where we live, and how we conduct our lives – the composition of the diet is probably the most important and most dominant factor which impacts our gut microbes."- Eran Elinav, MD, PhD Click To Tweet
That the diet influences our microbiome probably comes as little surprise. Some microbes prefer certain nutrient sources, providing them a competitive edge if those nutrients are present in their host's diet. Dr. Elinav explains that this edge can translate to downstream effects on the host, for good or bad, as seen with cardiometabolic health, for example. In response to dietary components, microbes not only modulate aspects of cholesterol metabolism, but they also produce a profusion of molecules, such as trimethylamine-N-oxide, short-chain fatty acids, and secondary bile acids, driving the pathogenesis of cardiovascular disease. In this way, the gut microbiome may predict cardiovascular disease risk.
And when global changes occur in a person's diet, such as from an animal-based dietary pattern to a plant-based one, the microbial population in the gut shifts to one that is more accommodating to the new menu. Although these shifts are somewhat predictable across large groups of people, each person's microbiome will respond in its very own unique way.
But microbial responses to individual components of a person's diet may influence host health, too. Dr. Elinav describes how evidence suggests that artificial sweeteners promote metabolic dysfunction, potentially increasing the risk for type 2 diabetes. Conversely, omega-3 fatty acids exert dramatic effects on the gut microbiome, which in turn elicits a range of beneficial responses, from enhanced immune function to reduced opioid-seeking behavior to improved mood, and many others.
When we eat might matter a lot, too — even from the standpoint of our microbes
"The timing of diet has an independent and peculiar effect on the composition and function of our gut microbes. Through these time-dependent interactions, our gut microbiome can independently impact our metabolic health or our propensity to develop disease."- Eran Elinav, MD, PhD Click To Tweet
But the discovery that the timing of our food intake influences the gut microbes left Dr. Elinav and his colleagues nonplussed. After sampling gut microbial populations every four hours during a single day, they learned that the activity and composition of a person's gut microbes change consistently (and predictably) across the course of a day – a phenomenon Dr. Elinav refers to as "stably unstable." If the microbes' diurnal pattern is disrupted, he says, the host has an increased risk of obesity and diabetes – diseases that are associated with altered sleep/wake cycles, such as those experienced with shift work. Normalizing the timing of food intake via time-restricted eating may help restore microbiome composition, potentially offsetting the increased disease risk.
Alterations in the gut microbiome drive recurrent obesity
"This post-dieting microbiome stored a metabolic memory of past obesity that predisposed the mice to an exaggerated weight regain the next time they were exposed to an obesogenic diet."- Eran Elinav, MD, PhD Click To Tweet
However, restoring the gut microbiome in the setting of obesity presents unique challenges. Dr. Elinav says that obesity has profound, deleterious effects on the gut microbiome, driving dysbiosis and impairing critical aspects of nutrient metabolism. Of particular concern is the inability to metabolize flavonoids, some of which, such as apigenin (found in parsley and chamomile) and naringin (found in grapefruit and cherries), participate in fat metabolism. This dysbiosis persists, even after weight loss, likely promoting recurrent (or "yo-yo") obesity.
The compounds produced during flavonoid metabolism are just some of the thousands of bioactive molecules produced by the gut microbes. These molecules, sometimes referred to as metabolites or postbiotics – are taken up in the gut and enter the body's circulation, where they can have far-reaching effects on multiple organ systems, says Dr. Elinav. In fact, half or more of the molecules circulating in our blood may have originated in or been modulated by the gut microbiome.
Intestinal permeability and metabolic endotoxemia
"[Gut] leakiness results in the influx of molecules from the gut into the sterile human body, which contributes to disease states or to exacerbation of disease in different contexts."- Eran Elinav, MD, PhD Click To Tweet
Of course, the organ most intimately associated with the gut microbiome is the intestine. Gut microbes secrete a milieu of compounds that simultaneously allow the passage and absorption of nutrients in the gut while maintaining a defensive barrier against pathogens, toxins, and harmful food components. Failure of this barrier drives "leaky gut" – also known as intestinal permeability – a condition in which gaps form between the tight junctions between the endothelial cells that line the gut. These gaps allow pathogens like bacteria or endotoxins (toxins that are released when bacteria die) to leak through the intestinal wall and pass directly into the bloodstream, driving both acute and chronic disease states, such as COVID-19, celiac disease, and neuropsychiatric disorders, among others.
Promoting gut health with prebiotics and probiotics
So how does one promote and maintain a happy, healthy collection of gut microbes? Two strategies – prebiotics and probiotics – merit attention, with varied scientific support.
Prebiotics are dietary components (primarily indigestible fibers) that promote the growth and survival of beneficial microbes in the human gut. Foods that contain prebiotics include asparagus, beets, garlic, chicory, onion, Jerusalem artichoke, grains, and breast milk.
Probiotics are live bacteria – either in foods or supplements – that, when consumed, promote or maintain a healthy population of gut microbes. Probiotic foods include yogurt, kefir, sauerkraut, and kombucha. But the evidence to support the use of probiotics is wobbly, at best, says Dr. Elinav, because the indigenous microbiome is openly hostile to newcomers, preventing their colonization, even temporarily, in the gut. As one researcher put it, "The attribution of efficacy to the consumption of probiotic microorganisms for health benefits has occurred in reverse order of the available scientific evidence."
Bacteriophage therapy: Exploiting a microbial arms race
"If [bacteriophage therapy] is successful, we would be able to really take a needle out of the haystack by targeting a single bacteria or a single type of bacteria without killing the entire microbiome and causing substantial collateral damage."- Eran Elinav, MD, PhD Click To Tweet
An exciting area of study in current microbiome research centers on bacteriophages, a class of viruses that infect bacteria. Bacteriophages are species-specific and typically only infect a single bacterial species or even specific strains within a species. For this reason, some scientists have posited that bacteriophage therapy may be a viable alternative to traditional antibiotics for the treatment of bacterial infections.
The microbiome is a fascinating and exciting area of research that is still in its infancy, with many questions as yet unanswered. In this episode, Dr. Eran Elinav and I discuss the gut microbiome and its role in human health and disease.
Scientists mentioned
Selected publications
- Personalized Nutrition by Prediction of Glycemic Responses
- Personalized Postprandial Glucose Response-Targeting Diet Versus Mediterranean Diet for Glycemic Control in Prediabetes
- Personalized Gut Mucosal Colonization Resistance to Empiric Probiotics Is Associated with Unique Host and Microbiome Features
- Potential roles of gut microbiome and metabolites in modulating ALS in mice
- Amyotrophic lateral sclerosis and intestinal microbiota—toward establishing cause and effect
- Hyperglycemia drives intestinal barrier dysfunction and risk for enteric infection
- Non-caloric artificial sweeteners and the microbiome: findings and challenges
- Environment dominates over host genetics in shaping human gut microbiota
- Transkingdom Control of Microbiota Diurnal Oscillations Promotes Metabolic Homeostasis
Learn more about Eran Elinav, MD, PhD
-
Circadian rhythm of the microbiome
-
How the circadian rhythmicity of the microbiome is regulated by food composition and timing. 1
-
The microbiome's role in regulating postprandial glucose response and how circadian rhythm disruptions, such as jet lag and shift work, may increase disease risk.1
-
How food timing and time-restricted eating may ameliorate microbiome-associated dysfunction in shift workers. 1
-
How our diet composition and timing are major factors that control the composition and function of the gut microbiota. 1
-
Lessons from hunter-gatherers
-
How the microbiome transforms to accommodate dietary changes, particularly comparing hunter-gatherer and Western diets.
-
How the microbiome is shaped in early childhood and remains stable across the life cycle, unless a person makes drastic diet or lifestyle changes.
-
The surprising finding that subtle environmental effects impact the microbiome, such as how the rainy or dry seasons influence the gut microbiomes of hunter-gatherers.
-
How drastic dietary changes, such as the switch between a vegetarian and meat-based diet, shift the microbiota toward an orientation better suited to the new diet, but these changes vary among individuals.
-
How a diverse microbiome may protect against metabolic diseases and how each person responds to diet in a unique way.
-
How urbanization profoundly reduces microbial diversity by almost 10-fold as compared to people living in hunter-gatherer populations.
-
Nurturing the microbiome in children
-
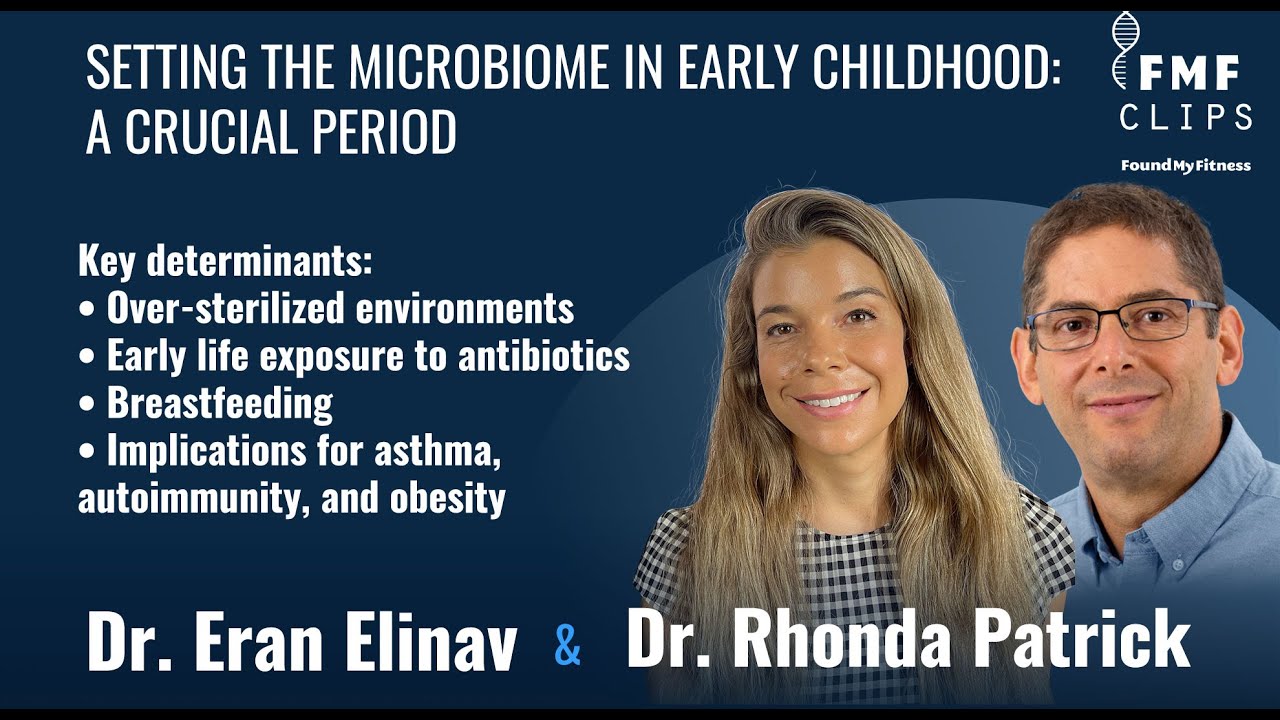
How the first three years of life are a critical period of development for the gut microbiome, and how parents might steward the configuration of their child's microbiome, shaping the risk of asthma, obesity, and other diseases in later life.
-
How an over-sterilized environment and exposure to antibiotics in early life may harm the microbiome. 1
-
The eye-opening connection between early childhood germ exposure and a reduced likelihood of allergies and autoimmune diseases. 1
-
The potential transgenerational effects of the loss of microbial diversity in the gut.
-
The contribution of genes versus environment in the microbiome composition. 1
-
Triglycerides and cholesterol
-
How the composition of the microbiome impacts blood lipids such as HDL cholesterol and triglycerides, along with anthropometric measures including weight, waist-to-hip ratio, and more. 1
-
Saturated fat
-
Whether a person's microbiome drives how they respond to saturated fat and the implications for nutrition research.
-
Artificial sweeteners
-
How food ingredients typically thought to be inert, such as artificial sweeteners and dietary emulsifiers, can impact gut microbes and ultimately influence human health. 1
-
How some artificial sweeteners alter the gut microbiota and disturb blood sugar regulation in mice. 1
-
How people vary in their response to artificial sweeteners such as saccharin that may be influenced by the gut microbiome. 1
-
How the gut microbiota likely influences a person's response to omega-3 fatty acid intake.
-
Recurrent obesity
-
Microbiome-related dynamics of weight regain and why some people have difficulty maintaining weight loss. 1
-
How transferring microbiomes from mice with recurrent obesity into germ-free animals induced obesity and type 2 diabetes, suggesting recurrent obesity results from a memory stored in the microbiome. 1
-
How one of the mechanisms of microbiome-associated recurrent obesity may involve dysregulated intestinal flavonoid levels, such as apigenin and naringenin, which usually help promote the burning of excess energy by adipose tissue. 1
-
How supplementation with post-biotics such as apigenin and naringenin, may be a strategy against microbiome-associated weight regain. 1
-
How fecal microbiota transplantation may restore the post-obesity microbiome, ultimately reversing the tendency towards recurrent weight regain.
-
Caloric restriction
-
The peculiar consequences of caloric restriction on the microbiome may account for some beneficial impacts on metabolism.
-
Intestinal permeability
-
How intestinal permeability, also known as "leaky gut," can allow microbes and their metabolites to translocate out of the intestinal lumen and influence the health and function of distant tissues, such as the brain, connective tissues, sometimes promoting a state known as "metabolic endotoxemia." 1
-
Approximately 50 percent of the small molecules found in human blood originate or are metabolized by gut microbes, demonstrating their intimate relationship with host physiology.
-
How bacterial products coordinate activity between the gut, mucus layer and immune system are integral to maintaining a healthy gut barrier.
-
How factors that promote intestinal permeability may vary from person to person.
-
The difference between prebiotics, probiotics, and postbiotics.
-
Why probiotics may benefit some people more than others. 1
-
How gut colonization with probiotics following antibiotic use, may in some cases, delay the natural recovery of the indigenous microbiota, possibly to the detriment of the host.
-
How precision or "next-generation" probiotic therapy, where probiotic perturbation and supplementation are tailored intelligently in response to an analysis of a person's existing microbiome, may ultimately replace current probiotic strategies and improve health and disease.
-
Whether stool samples are an accurate assessment of if a probiotic has colonized the gut.
-
How bacteriophage therapy may be a tool to precisely perturb the microbiome, eliminating pathogenic species while leaving commensal species unscathed and circumventing antibiotic resistance. 1
-
How bacteriophages combined with probiotics could generate targeted personalized therapies.
-
Challenges and timelines as to when next-generation therapies like bacteriophages and precision probiotics begin to take off. 1
-
TMAO
-
How the ability of a person's microbiome to promote the production of trimethylamine-n-oxide (TMAO), as a byproduct of L-carnitine and choline metabolism, may influence their heart disease risk. 1
-
Weight gain from smoking cessation
-
Smoking cessation-associated weight gain and how chemicals from cigarette smoke penetrate gut tissues, influencing the microbiome. 1
-
Tips for diet personalization
-
Whether there are any generalizable ways to maintain a healthy gut microbiome.
-
The DayTwo clinical commercialization and licensing of the personalized nutrition algorithm built by Dr. Elinav and his colleagues at the Weizmann Institute in Tel Aviv.
-
How continuous glucose monitors and data-driven diet personalization can often lead to durable insights that result in lifestyle adjustments that no longer need constant monitoring in order to be useful and applicable. 1
-
How to reproduce some of the more rudimentary observations of the more advanced diet personalization algorithm using a standard glucometer.
-
Dr. Elinav's perspective on the utility of continuous glucose monitors for the purpose of diet personalization and self quantification.
Dr. Patrick: Hello, everyone. Welcome back to the podcast. I'm here today with Dr. Eran Elinav. He is an M.D., P.H.D, and a professor of immunology. He is the principal investigator of two labs, one at the Weizmann Institute of Science in Tel Aviv, Israel, and the other at the German Cancer Research Center in Heidelberg, Germany. His research focuses on understanding the complex interaction we have with the bacteria in our gut and how this shapes human health and disease.
Dr. Elinav along with his collaborators discovered that people consuming identical foods have diverse metabolic responses that depend on a variety of factors, including microbiome composition. He also discovered along with his collaborators that bacteria in our gut are on a circadian rhythm and how this may have metabolic consequences that depend on when we eat in addition to what we eat.
I'm very excited. We're going to talk about a lot of interesting stuff, all things microbiome today. I kind of want to start off, Eran, with the circadian rhythm that the gut microbiome is on and how this can relate to meal timing and metabolic responses. We have talked quite a bit about circadian rhythms on the podcast from, you know, the master circadian clock in our suprachiasmatic nucleus and how light resets that clock and how there's peripheral circadian clocks in other organs such as the liver and how food intake is the major signal that resets that clock. So a few years back, your lab discovered that the bacteria that reside in our gut have their own circadian rhythms. So can you talk...maybe just explain a little bit about this to people?
Dr. Elinav: Absolutely. It's great to be talking to you, Rhonda. We have done a lot of research, I mean, trying to understand better how the composition of diet impacts our gut microbes and through interactions with our microbes mediate metabolic health and metabolic disease. But surprisingly, we stumbled upon a quite major discovery in which not only the composition of diet impacts our gut microbes, but actually, the timing of diet has an independent and very peculiar effect on the composition and on the function of our gut microbes. And through these time-dependent interactions, our gut microbiome can independently impact our metabolic health or our propensity to develop diseases such as obesity and type 2 diabetes.
And basically, the discovery came across a very laborious project in which we tried to characterize the composition and the function of our gut microbes at different time points along the 24-hour cycle. So basically, my students sampled mice or humans each...every four hours of an entire 24-hour cycle, and then we were surprised to find that many of the functions of our microbes change in very consistent manners along the course of a day. Now, this was super surprising to us because, if you think about it, our gut microbes live completely in the dark. So how do they know that it's day or night and change their activity so reproducibly at the exact same hours along a 24-hour cycle? This led to three years of intense research, and the answer was that our microbes sense the timing in which we eat or do not eat and change their activity accordingly. In other words, during the day when humans are awake and eating, the microbes behave in one way, but during the night when we're asleep, they behave in very different manners. And in mice, which are awake at night and sleep during the day, this activity is completely opposite.
Dr. Patrick: So I have a follow-up question for you. You know, there's been a lot of research that has looked at how many genes in our body, and particularly genes that relate to metabolism, are controlled, you know, by a circadian rhythm. And so, for example, you know, there've been quite a few studies now that have shown that people are...if you give them identical foods in the morning versus the evening time, and you look at postprandial glucose response, for example, you'll see that people...you know, their postprandial glucose response is much higher in the evening, people are more insulin sensitive in the morning as well. So, to the bacteria in our gut, is there a role that they play in energy production in perhaps the postprandial glucose response, for example?
Dr. Elinav: Yeah. That was one of the most surprising and intriguing parts of our discovery. Not only did we discover that the timing of our diet impacts the composition and the function of our gut microbes throughout the course of a day, we found that this amazing tangle between our diet and our microbes also signals to the host, to mice in some cases and to humans in other studies which we conducted. And basically, this circadian microbial activity builds into the circadian clock which hallmarks every cell and organ in our body. In other words, the microbial circadian rhythmicity is a critical part that participates in disorderly diurnal behavior of our cells and our organs at different locations in our body. And once we disrupt the circadian microbial activity, for example, by changing the patterns of our diet or by subjecting mice to jet lag behavior, the microbes go crazy and stop behaving in this orderly manner throughout the course of a day, and this directly reflects on how the host behaves in its normal circadian behavior. And we found that once we disrupt the microbes, the host is now susceptible to develop obesity and type 2 diabetes, which is exactly the set of diseases which hallmark humans which feature a chronic disturbance in their wake-sleep patterns such as shift workers that are at a substantial risk of developing obesity and type 2 diabetes. And for many years, we didn't know what was the missing link that caused this risk behavior, and now we think that at least part of the answer lies within the microbes themselves.
Dr. Patrick: Do you think that there's any potential solutions for, for example, shift workers who are awake in the evening hours and eating food? So we've learned a lot about time-restricted eating or time-restricted feeding and how that can potentially positively impact, you know, a shift worker's metabolism if they try to limit their food, for example, into a certain time window, maybe 10 hours, you know, rather than, you know, eating throughout the time that they're awake at night. Do you think that this also has implications for affecting the gut microbiome as well, doing this time-restricted eating if you're a shift worker or even in general?
Dr. Elinav: Absolutely. And what we've discovered at least in mice, and also to some extent, we and others have discovered this to occur in humans, is that the dominant factor that determines the diurnal activity of microbes throughout the course of a day is the timing of our feeding. And when we disrupt the timing of our feeding, for example, by subjecting mice to a shift to our kind of lifestyle or jet lag or even in genetically clock-deficient mice, we disrupt the microbial circadian activity. However, if we take all of these disrupted conditions and now we time-restrict the feeding of these mice to imitate the normal eating behavior in non-disrupted mice, then we can completely restore the microbiome circadian activity and its effect on the metabolic and immune function of the host.
So at least in mice and to some extent in humans, indeed, time-restricted feeding could restore unaltered microbial behaviors across...uh, behavior across a course of a 24-hour cycle. However, you know, if you think about it, this does not really solve the human problem because, if a doctor or a physician or a nurse in a hospital has to go through a night shift and therefore features a disrupted microbiome and a risk of developing obesity and type 2 diabetes because of the disrupted microbiome, you cannot ask a nurse or a physician, you know, to eat after they've been awake for an entire night just so they restore their gut microbiome composition function. So what we're trying to do is to decode the molecular mechanisms by which our microbes communicate with our host cells at different time points throughout the course of a day. And when we understand what goes wrong, what gets disrupted when the circadian rhythm is disturbed, maybe we could develop new interventions that would enable the microbes to now correct the signal to the host and to avoid these risk behaviors and these susceptibility to disease.
Dr. Patrick: If some of these microbes...so they're obviously sensitive to the feeding-fasting period, so food intake versus not eating. What about the composition of the food? Like, does that play a role? Does that matter in addition to, you know, some of these microbe species that are, you know, active on their diurnal circadian rhythm?
-Dr. Elinav: I think that of all the different environmental factors that affect us humans and surround us, our stress levels, the medications we take, where we live, and how we conduct our lives, the composition of the diet is probably the most important and most dominant factor which impacts our gut microbes. And this has been shown by us in the Personalized Nutrition Project, but it has been extensively shown by many others. And I think it is safe to say that of all the features that we and others are studying, there's nothing more important and dominant than the composition of our diet.
Dr. Patrick: Let's talk a little bit...dive into that a little bit like, you know, maybe starting with some of the macronutrients. Like how do the composition of our diet including proteins or carbohydrates...complex carbohydrates versus, you know, simple carbohydrates, or even fat and the type of fat, saturated fat versus polyunsaturated fat or monounsaturated fat, how does that affect...or whether we eat a plant-based diet or animal-based diet. How does that affect the composition of our microbiome?
Dr. Elinav: That's a great question, and to be honest, I think that our very young field is only beginning to mechanistically unravel these complex effects. And there are many different types of effects by which micro and macronutrients in our diet could impact our microbes. For example, some of our nutritional inputs could serve as an energy source to microbes, and some microbes would preferentially digest some but not other components of our diets. As you may imagine, there may be a competitive advantage to some microbes over others depending on the diet that they're exposed to. Other impacts could relate to an even more enigmatic part of the microbiome which is bacteria-bacteria communications. And we increasingly know that we have trillions of bacteria in our gut and these form ecosystems or communities in which they are very marked and poorly understood communication channels between different bacteria that determine who would survive, who would flourish, and who would not. And these also in many interesting aspects relate to signals that are obtained from our diet. And a third example of how diet composition could impact our microbes relates to the host, and many of our dietary components are sensed or are absorbed by the host, which changes its behavior in response to these compounds. And the host could be, you know, regarded as a very sophisticated incubator that houses all of these microbes, and by changing its behaviors or its conditions, the host in response to diet could change the relative composition and function of different microbes over others.
Dr. Patrick: There are people, you know, living in, you know, certain parts of, for example, Africa that are...you know, they eat a very, very...like from day to day, the diet's very similar. You know, they're eating lots of complex carbohydrates, for example. I mean, it's a very much...they eat the same meals like almost every day versus someone living in like, you know, in the Western world in the United States, for example, where there's...you know, the diets vary so much from person to person and depending on like processed foods versus, you know, eating whole foods, you know. How stable is the microbiome if, for example, we were to switch diets, so if someone in the Western world was to eat something more like, you know, plantains and these, you know, complex carbohydrates and vice versa?
Dr. Elinav: Yeah. It's an excellent question. And the answer is that it really depends on which resolution you're looking at the microbiome in terms of its stability. So just to give you an example. If you look from a bird's eye view way from up top at an adult person's microbiome, and you're talking about a healthy adult with a relatively stable lifestyle, then the microbiome composition over time in that adult would seem very, very stable. We discovered that even in industrialized nations, people are usually exposed to no more than 40 or 50 components of dietary composition in a routine lifestyle. So the variation in our individual exposure to food is much lesser than you would expect. And if you just, you know, look into the composition and the function of the microbiome from this resolution, you would find that it changes in a very minor manner between the age of three until we get to an old age or if we develop disease or change our lifestyle. However, if you dive deeper into the microbiome, you would find that there are more interesting changes that are, you know, more subtle and characterize our healthy being.
For example, there are studies in Africa looking into rainy or dry seasons, which are characterized by different exposures to different crops and different foods, and you can see that there's a very consistent and very reproducible change that is based on the changes in these seasons and what they represent. If you look even closer as we just discussed, even in the 24-hour cycle, you would find that the microbiome is what we call stably unstable. It is stable but it oscillates throughout a 24-hour cycle in a very reproducible manner, and this relates to a healthy state. Now, when you start adding into it all the perturbations and all the exposures that a human may be experiencing, for example, changes in diet as you highlight, changes in where we live, changes in our health status, in our stress status, in the medications that we eat, all of these environmental signals or cues reflect on our gut microbes in a way that may impact our physiology or risk of developing diseases.
Dr. Patrick: What do you think about, for example, a vegetarian diet versus someone...? It's become quite popular actually in the United States, this carnivore diet where people actually cut out all carbohydrates and they eat only meat. How is that going to impact the microbiome, or do we know?
Dr. Elinav: Yeah. There are very elegant studies early on from kind of birth of the field by researchers such as Jeff Gordon and Fred Bäckhed, which have shown that, you know, if you abruptly change the composition of the diet from one type to another, for example, from a veggie to a carnivore diet, you very reproducibly change in an average...in a population average, you very reproducibly change the composition of the microbe into one which accommodates better the new diet. And this is kind of when you look at a relatively low resolution into the microbiome. However, if you look at a higher resolution, and this I think was one of the exciting discoveries that we came across in the Personalized Nutrition Project, you would find that even within the same diet, people react very differently when you look closely enough. So the answer is a complex one.
You know, in 2015, we've conducted our own kind of mini-trial in which we took a group of healthy human individuals, and we've asked them politely to eat only white rice for a week, then only, you know, a steak for another week while we extensively measured them for their microbiome. And we found indeed that the microbiome changes in a very reproducible manner. Even if the starting configuration is different between people, the direction of the change is very similar when you look at the same bacteria in different people with respect to the response to the same dietary change. But when you look at a more real-life scenario, you would find that people are uniquely responding to dietary components even if they're exposed to the same exact diet, and this is the hallmark of the Personalized Nutrition approach.
Dr. Patrick: What role does microbiome diversity play in, for example, the, you know, metabolic responses to food like postprandial glucose response or, you know, when it comes to our personalized responses to diet?
Dr. Elinav: Well, if you're looking at...or if you're thinking about microbial diversity or the richness of a given microbiome, there are many interesting observations that are trying to relate the loss of diversity to a propensity to develop disease. So, for example, if you look at indigenous populations of humans, hunter-gatherers and so on and so forth, you would find in some studies that the diversity of the microbiome can be tenfold higher than the average diversity that we can see and measure in the same human beings when they live in modern "societies or industrialized societies." And people have tried to link this amazing reduction in diversity to the modern risk of developing diseases such as obesity, type 2 diabetes, fatty liver, and even cancer, and other diseases. However, the causal role implicating the richness per se of the microbiome to these diseases still merits further investigation. So the jury is still out there. Although I must say that in many microbiome-associated diseases, we indeed see a reduction in diversity that characterizes these disease states. Whether the diversity reduction is by itself a risk factor to the development of disease, or whether it just reflects the emergence of dominant disease-causing microbes is an open question that at least to my opinion has not been sufficiently answered yet.
Dr. Patrick: Speaking about the diversity and how it changes...the question is how it changes throughout the lifespan. So you mentioned, you know, a few minutes ago about the microbiome being pretty stable, generally speaking, at...you know, after the age of about three. This is a two-part question. One would be, you know, what factors...? Like it seems as though during early development, it would be very important, you know, if you're shaping the overall general stability of the gut microbiome in the first three years of life, what impact, you know, for example, feeding your infant and young child, you know, breast milk, which has things like human milk oligosaccharides and a variety of factors that have been shown to be very important for the gut microbiome in shaping it, and then also, you know, what foods you do feed your child or antibiotic use or factors like that. Do you think that parents should be focused somewhat on the health of their young growing child's, you know, gut microbiome in those first three years of life, or exposing them to, for example, you know, soil and other, you know, bacterial exposures that they're getting from their environment?
Dr. Elinav: I think that the data we have certainly points toward that direction. So there's lots of data emerging in animal models and also quite a lot of data emerging in humans that suggest that the critical window of opportunity in the first three years of a human's life is the window in which we shape our adult configuration of the microbiome. And this window of opportunity is also a window of risk is one in which, you know, the microbiome can be influenced both by our parents and our immediate surroundings but also by what we eat, what we're exposed to, and the amount of environment that our microbes sense. And this kind of brings a little bit of a paradox because we as humans were raised in the last two centuries to be afraid of microbes and of infections, which justifiably were the leading cause of death in humans for millions of years. We now are slowly realizing that by overly protecting our children from exposure to these microbes that surround us in, you know, every material that surrounds a young child may predispose to an underdevelopment of their microbiome. In other words, by subjecting kids to an overly sterile condition, we may be harming them by not allowing their microbiome to shape in a diverse enough manner that would train our immune system and would impact our healthy metabolism in a way that would result in health in years to come, especially in mice but also to some extent in humans, it was shown that early-life exposure to antibiotics, for example, could save lives in many cases, but the price that we may pay is an increased risk for diseases such as asthma, these are elegant studies that were performed by my friend and colleague, Brett Finlay, and to obesity in later life and other diseases. So, you know, the proof of causality especially in human patients is very hard to achieve, but it seems that the majority of evidence from the decade and a half of microbiome research certainly points to that possibility and to that direction.
Dr. Patrick: I remember...my son is now four, but, you know, when I was, you know, a really new mother, I remember coming across a study where early life exposure within the first year to dirt like, you know, dirt and, you know, obviously the bacteria that are in the dirt, it seemed to be protective against later development of asthma was a big one. I think there was, you know, an autoimmune type of, you know, response. As you mentioned, you know, we... This hygiene, you know, obsession that we have in the industrialized nations, you know, which, you know, there's a good rationale behind that, but, you know, we all live in these buildings, and, you know, not many people have, you know, any dirt or trees or just, you know, sand, and so you really in some cases have to make an effort to go out and expose your young child, you know, let them play in the dirt, let them get dirty. And so I definitely tried to do that as much as possible when my son was...during early development.
Dr. Elinav: I totally agree. And this is supported, for example, by epidemiological evidence of some of the autoimmune or other inflammatory diseases being much less prevalent in kind of, you know, "dirtier countries" or countries in which the prevalence of exposure at early life to environmental infection is higher as compared to cleaner "countries," which suffer from a marked increase in these autoimmune or other inflammatory diseases. There are very elegant studies by my colleague, Martin Blaser, from NYU showing in mice and I think also in humans that this overly...these distinct depletion or changes on the development of the microbiome could impact on the susceptibility to develop diseases such as inflammatory bowel disease in later life. So this link certainly has been suggested and to some extent has been demonstrated to probably occur. A formal proof of causality in diseases, which may take many years and even decades to develop, is very hard to achieve in humans. So there too I think that the supporting evidence is very robust, but in order to get a completely, you know, finalized proof, you know, it will take more time.
Dr. Patrick: The question I have is like is there...? We're talking a lot about the environment, how that's shaping the gut microbiome, and it just sort of came to my mind like, you know, there are some women, for example, that, you know, have IBD, irritable disease, or something for whatever reason, you know, whatever the causal factor is. Is there a genetic component or something that can still influence the microbiome composition let's say that woman has a child? And, you know, like is there some sort of transgenerational effect of microbiome? Like what if, you know, this woman had a lot...? Maybe she's got IBD because she had serial exposures to antibiotics combined with, you know, poor meal timing or, you know, who knows what the combination of environmental factors could have been to influence her microbiome. Does the mother's microbiome affect the child's microbiome? Is there any evidence of that?
Dr. Elinav: Well, I would divide my answer into two parts. First of all, every child is born sterile to the best of our knowledge and acquires his or her microbiome during the neonatal period from his or her immediate surrounding, which mainly consists of their parents who are very close to them. So in addition to many other environmental factors, it seems that a child's microbiome is very much influenced by that of their parents, and especially their mother in cases in which the mother, you know, takes more care of a baby than the father. With that said, the question you're raising is a fundamental question in the microbiome field, which if I were to rephrase would ask whether the microbiome is shaped by our genes or by our environment. And this remained an open question for many years until we conducted an ambitious study in which we took 500 healthy individuals, and we comprehensively profiled their microbiome and assessed as much as we could many of the environmental factors that influenced them including their dietary habits and so on and so forth. And we sequenced their genes, so we characterized their human genome. So for the first time, we could directly compare the influence of our human genome and our environment on the composition and the function of the microbiome and also to compare the potential contribution of the microbiome and the human genes on different human traits. And the answer was an intriguing answer. What we found was that most of the effect shaping our microbiome comes from the environment. Only 1.9% of the variability in the human microbiome could be explained by differences in the human genes, while close to 99% of the variability in the human microbiome was explainable by factors coming from people's environment. That doesn't mean that the 1.9% of the genes is not exceedingly important. There could be some genes there that are exceedingly and dramatically important in generating a healthy microbiome. It just tells you that the weight of the effect is mainly coming from the environment, and this is very encouraging because the environment in contrast to our human genes could be modulated. So if a microbiome changes for any reason to a configuration which favors disease, we could hopefully find ways by which we modify the environment that is sent by the microbiome in order to reverse it back into a healthy configuration.
The second revelation from this study was equally interesting to us, and what we found was that some human traits were only impacted by the human genes. So, for example, if you look at human height, it is not affected by the microbes whatsoever. So almost all of the explanations for differences in human height came from the human genes and not from the microbes. However, when we looked at a number of metabolic parameters such as weight, waste to heat ratio, cholesterol levels, and many other metabolic features, we found that the microbes...the microbiome and the human genes had independent and very substantial effects on these traits. In other words, the microbiome in the human body or the human genomic system participate in the determination of our healthy metabolism and our risk of developing metabolic disease.
Dr. Patrick: You brought up a question... I wanted to circle back to dietary composition because you've done so much work on that but that what you just said brought up a question in my mind about cholesterol, the microbiome having an independent effect on cholesterol. We do know that genetics plays a role as well. Have you or any of your colleagues looked into the mechanism for that or multiple...I guess probably multiple mechanisms? I'm kind of thinking along the lines of even just inflammation and how, you know, when there's an inflammatory response, you know, cholesterol is kind of produced. Like that's kind of a well-known thing is that you should always have at least an "N of two" when you're getting your cholesterol levels measured because, you know, if you have some sort of stressful event or something that's causing inflammation or if you're sick, you can have, you know, high cholesterol levels. And that's not necessarily indicative of what your cholesterol levels are.
Dr. Elinav: You're absolutely right. And I can tell you that we and several other groups have reproducibly found that different aspects of healthy cholesterol and fatty acid metabolism in humans and in mice are modulated by the gut microbiome. So, for example, in the Personalized Nutrition Project and in interventional trials that were followed as part of this project, we found that modification of the personalized nutritional recommendations could lead to an improvement in HDL cholesterol, the good cholesterol.
Another group from the UK conducted a very ambitious follow-up trial similar to the Personalized Nutrition Project which we started with called the PREDICT Trial, and in this trial, they could show something very exciting, which is that the microbiome and the host could be used to predict a person's triglyceride levels. In other words, not only did they associate the microbes to features of fatty acid metabolism or triglycerides, which are one of the risk factors for cardiovascular disease, they could use data coming from the microbiome in order to predict a person's levels of triglycerides, which provides another stronger proof to the possible causal association between the two features.
Dr. Patrick: Do you think that some of the confounding factors in the many, many studies that have been done, for example, on saturated fat and, you know, the role of saturated fat in cardiovascular disease risk or in certain biomarkers that indicate cardiovascular disease risk like high cholesterol, LDL cholesterol, for example...? There's conflicting data where it's not always bad, but it does seem to be bad. So is there a microbiome component like in the way your body responds to saturated fat?
Dr. Elinav: I think that, you know, the specific answer is I don't know, but the conceptual answer is that in every study that we look or conduct, we find that inter-individual differences in the microbiome could play a role and potentially explain variabilities between studies in their outcomes even if they seemingly do the same thing and reach different conclusion. And I can give you endless examples. For example, our own studies on non-nutritive sweeteners or artificial sweeteners suggested that the microbiome is a very major player that modifies the response of some people but not of other people into some of the nutrients. And if you look at the body of evidence suggesting that nutrients adversely or favorably impact the human body, you know, it's all over the place, and the results are very conflicting with each other, and people, you know, spend their career fighting with each other, while some of the explanations could lie within inter-individual variabilities in their physiology including ones that are related to their microbiome.
Dr. Patrick: Since you mentioned the artificial sweeteners, maybe we can kind of dive into that just for a moment because it's fascinating work that your lab has done on the metabolic effects of, for example, artificial sweeteners but also like food additives, emulsifiers. So you mentioned that the people had diverse responses to artificial sweeteners. What were those responses?
Dr. Elinav: Yeah. So the study that we've published mainly focused on mice, and in mice, we studied several artificial sweeteners, but we mainly focused on saccharin as the very marked example. And what we found to our very big surprise was that mice featured a counterintuitive disturbance in their glycemic responses when they were exposed to saccharin, and this was driven by their microbiome. So, for example, when you exposed mice to saccharin at different doses and took the microbiome after this exposure and transferred it into germ-free mice that never saw saccharine, they developed the same disturbances in blood sugar control as those of the donor mice. And so this was a very complex study that provided a proof of concept that some dietary compounds that we use, mainly modern dietary compounds that we regard as inert because they don't seem to directly impact our body, may impact our body in peculiar ways indirectly through their effects on the microbiome. And this I think proof of concept study was followed by many other studies. You mentioned emulsifiers, and there were studies on food colorants and other ingredients, which may bear effect in some people based on their impacts mediated on the gut microbes, and this needs to be taken into consideration when assessing the safety and the inertness of such substances.
Dr. Patrick: I think I recall reading the levels of the dietary emulsifiers were even perhaps in levels that were relevant for humans.
Dr. Elinav: Yeah. In some of the mouse studies, the levels were higher than in humans, but in many of them, the levels were, you know, very similar to the ones observed in humans. It's difficult to directly compare mice to humans. The metabolism is not exactly the same, and we need to, you know, say that out loud. The concepts in many cases are very similar and the effects are very similar, but mice are not humans. But at least they suggest that such impacts could be happening. And, you know, the burden of proof is on us. You know, before we recommend a substance, we need to make sure that at least sufficiently we understand what it does to our microbes, what it does to our human body to make sure that we do no harm. So not every mouse-based study could be directly translated into humans, but many of them provide an intelligence hypothesis that needs to be ruled in or ruled out in human studies that follow.
Dr. Patrick: Well said. Were there any preliminary human studies that were followed up with the artificial sweeteners and/or the emulsifiers?
Dr. Elinav: So with the artificial sweeteners as part of the original study, we published a very preliminary small scale study suggesting that personalized responses to saccharine in humans do occur and could be even transferred upon microbiome transfers from humans into germ-free mice. This was a very small preliminary study that we and others are trying to follow up on in larger controlled trials that I hope would teach us on potential personalized effects and how we can anticipate them or predict them in ways which would keep the use safe while, you know, letting people enjoy sweetness. But I would certainly say that there is emerging evidence that the findings that we came up with are not only reproducible in multiple animal models, you know, starting from flies and all the way to mice, rats, and piglets, but they may be relevant to humans. The jury's still out there. This is a very young field. We'll wait for more results.
Dr. Patrick: Oh, if I can give you a suggestion, I think most people nowadays don't consume saccharin like they did 30, 20 years ago. The big ones that I know a lot of people would be interested in knowing whether or not they're affecting the microbiome in a good or bad way or if they're neutral in their effects on metabolisms as well would be some of the non-nutritive sweeteners that are from natural sources like stevia or the monk fruit extract. So if you guys are interested in looking at that, I think, you know, that many people would be very, very interested in that data as well because a lot of people consume it.
Dr. Elinav: I can only say we're on it. Stay tuned.
Dr. Patrick: Great. So we've been talking quite a bit about obesity throughout the podcast and how the gut microbiome's affecting obesity. And I want to kind of dive into that a little bit more, but before we get there, I just have one question. I'm not sure it directly relates to your research. I know you've written about it in really well-done review articles that you've published. The effects of omega-3 fatty acids on the gut are super interesting. I'm a big omega-3 fan, and I remember reading, you know, a couple of studies coming out that were very quite surprising to me how the omega-3 was affecting the gut microbiome in what I thought to be a positive way.
Dr. Elinav: Yeah. You know, omega-3 is a classical example of a compound which has been suggested to be very beneficial to the human body in different manners, but it also bears surprising impacts on the gut microbiome. And this needs to be taken into account in assessing the overall effect of these compounds on different people because different people have different microbiomes. And so the effects on the different people's microbiomes could determine the different outcomes upon consumption of omega-3. You know, these are not my studies, but I can only tell you that in every single example of a food component or food additive that we've tested, and we've tested thousands of them in over 100,000 people that underwent our Personalized Nutrition pipeline or project, we found that people distinctly react to foods or food components or food additives even if they're exposed to the same exact amounts of the same exact component, and this includes fatty acids such as the ones that you've been asking about. And, you know, we don't know exactly what the complex array of causes are that determine this individualized responsiveness to different foods. This is a huge black box which we bypassed computationally, but it seems to involve both factors related to the host, factors related to our lifestyle, and factors related to our microbiome.
Dr. Patrick: So it definitely seems like, you know, understanding more of these environmental factors including the microbiome composition is extremely important especially for, you know, sort of illuminating the conflicting data out there for, you know, you'll find all these studies where one thing's good for you, but then another study says it's bad for you. It's definitely a lot more complicated than we originally thought. But I want to kind of shift gears and talk a little bit more about obesity because you had a very recent study actually that was very interesting and looked at some of the potential mechanisms behind why people that are starting out overweight or obese go on some sort of dietary program to lose weight, and yet they tend to gain that weight back quite easily. So can we talk a little bit about some of that recent work?
Dr. Elinav: Absolutely. This phenomenon which is medically called recurrent obesity but is more widely known as yo-yo obesity characterizes up to 80% of all obese individuals worldwide. So this is the most common pattern of obesity that we know of yet we have very little clue on what drives it. The pattern is as you suggested, a person who gains weight for any reason and then goes on in one of many different diets that are out there. And most diets are very efficient in reducing weight in the short term because they involve caloric restriction. So that person diets on one of these many diets, loses weight back to his or her original low body weight, but then within 12 months of successfully dieting, 80% of people go on to redevelop obesity or regain all the weight that they've lost and even regain a little bit more than they originally had. And from cycle to cycle of obesity and dieting attempts, we seem to get more and more obese until we gain...we become formally obese. This is exactly the pattern of recurrent or yo-yo obesity.
Now, if we're trying to...or if we're starting to scrape the surface in understanding the molecular mechanism that drives obesity...we know very little about this driving...about this recurrent yo-yo obesity phenomenon. So we tried to study it in mice, and we developed three or four different animal models that recapitulate this recurrent obesity behavior in humans. In other words, for example, we took mice, we put them on an obesogenic diet, we gave them a diet rich in fats and sugar, they gained weight, then we switched them into a low-fat diet. They dieted back to their original level. And then we re-exposed them for a second and a third and a fourth cycle. And what we could see was exactly the same phenomenon that is observed in humans. From cycle to cycle, mice seemed to regain more and more weight even when they started from the exact same weight as never obese mice and were exposed to the same exact diet. This is the exaggerated weight regain that characterizes yo-yo obesity in humans.
Now, in order to try and study and understand what the drivers of these behaviors are, we looked into tens of different parameters that can be measured in mice after they successfully diet to look for something in the mice following a successful diet that could store a bad memory of their previous obesity. And it seems that everything seemed to normalize after a successful diet. All the hormonal and the endocrine and the metabolic features that we could measure totally normalized after a successful diet other than the gut microbiome. When we measure the gut microbiome, it seemed to be persistently disturbed as though the mice were never dieted. It had a configuration which was very similar to the one that we observed during obesity. And when we took this microbiome that never normalized after a successful diet and transferred it into germ-free mice, these mice developed obesity and type 2 diabetes, meaning that this post-dieting microbiome stored a metabolic memory of past obesity that predisposed the mice to an exaggerated weight regain the next time they were exposed to an obesogenic diet. And when we probed even deeper into this memory microbiome, we found that it induced this trait of exaggerated weight regain by altering its ability to degrade dietary compounds which are called isoflavonoids. Normally, we found that isoflavonoids from diet are degraded by the microbiome to compounds which swim into adipose cells and signal to them to release more heat and store less fat when we are exposed to an obesogenic diet. But when these compounds were missing after a successful diet, the adipose cells, the fat cells were no longer given the signal to release heat and not to store fat, and now they were storing more fat and making the mice more obese as compared to non-yo-yo obesity mice. In other words, the microbiome was driving this exaggerated weight regain tendency by changing its metabolism of distinct molecules coming from our diet.
Dr. Patrick: If I remember correctly, some of these distinct molecules were these flavonoids like apigenin, which is high in, for example, parsley and naringenin or naringenin. I don't know how you pronounce it, but it's from grapefruit really essentially. So there's bacteria that are degrading or metabolizing these flavonoids. The obesity is causing a decrease in these types of bacteria. Is that correct?
Dr. Elinav: Exactly. So the bacteria normally...actually generate these flavonoids for more complex flavonoids by chemically modifying them. Once this obesity and then successful dieting occurs, the change in the microbiome that is so persistent means that the microbes are no longer able to generate these compounds, and this leads to a cascade of events that results in more fat accumulation per given diet leading to exaggerated weight regain.
Dr. Patrick: So do you think then that perhaps consuming foods high in some of these compounds like grapefruit and/or supplementation would help with that if your microbiome is not producing those compounds but you need those compounds? Is that correct? Am I understanding that correctly?
Dr. Elinav: It sounds like the microbiome is not producing the compounds if the microbes that are degrading these compounds are expanded in these obesogenic conditions, and therefore there's more degradation of these compounds and less of them that survive this microbial activity. And indeed at least in mice, what we've found was that if we intervened by resupplementing a mice with these now missing metabolites, we could avoid or treat exaggerated weight regain and obesity that it induces. A different approach that we've used in mice and seem to be highly effective is the replacement of this bad memory microbiome with a microbiome that had the ability to generate the right compound. And by fecal microbiome transplantation at least in mice, we could reset the mice to not develop this yo-yo obesity phenotype. So, it seems that by understanding the molecular mechanism that drives obesity in this distinct state, one could intervene through the microbiome or at least in mice and reverse this tendency and therefore treat obesity or ameliorate obesity at least in this context.
Dr. Patrick: Do you have any plans to look in humans, for example, that you could give them a supplement with these flavonoids to see if that...how that affects the metabolic outcome?
Dr. Elinav: It's not only plans, it's an ongoing trial that is aimed at utilizing the many pipelines that we've developed in mice to measure these effects and to measure the possible microbiome impacts on recurrent obesity and to study them in humans. Of course, humans are a much more complex animal than mice, but many of the concepts seem to hold also in humans. So we're studying this and trying to understand what happens in humans, which are the bugs and the molecules that they secrete which may contribute to this bad microbial memory that we have identified in mice, and how we can intervene in humans through different approaches including metabolites supplementation that would reverse or treat recurrent obesity in humans.
Dr. Patrick: That's very exciting. I eagerly wait for the data. You mentioned that people that are...you know, can successfully lose weight by a variety of diets, and a lot of them have to do with caloric restriction, in other words, they're just eating less food, and how, you know, that helps with weight loss. What effect does caloric restriction have on the gut microbiome?
Dr. Elinav: I think that it's very interesting to note that just like the findings that we and many others after us have noted with respect to recurrent obesity and its effect on the microbiome and downstream metabolism, equally interesting studies that recently came out that suggest that caloric restriction may have a peculiar effect on the microbiome that may drive its beneficial effects. There are many studies suggesting that periodic food restrictions such as, you know, those 16:8 diets and many others may have beneficial metabolic effects, although, you know, the jury is still out there. I need to be careful. Some studies have been showing less impressive results. But at least some of the effects may be mediated by the microbiome. Certainly, we see in these studies that caloric restriction or periodic avoidance of food has distinct changes on the microbiome, and these may contribute to different metabolic outcomes that are measured in these studies.
Dr. Patrick: I want to talk a little bit about...you know, we just touched on it a moment ago about our gut microbiome being these little factories that are turning out different compounds and metabolites. I think it's referred to by you and others as the microbiome metabolome.
Dr. Elinav: Yep.
Dr. Patrick: Can you talk about maybe just a couple of, you know, their producing compounds that could be beneficial in some cases as we've talked about, but also compounds that may not be so beneficial?
Dr. Elinav: Absolutely. And there are increasing evidence suggesting that one may regard our microbiome among many other descriptions as a biochemical factory that generates or modulates many thousands of small molecules that could be potentially bioactive and are called metabolites. And what we find super interesting about these metabolites or these small molecules is, A, that they have, in many cases, a peculiar chemistry, you know, that we've not recognized before. And B, that these molecules, in contrast to the microbes that make them, can influx, can swim into our sterile body where they can reach a very distant cells and organs and impact them. And by understanding the unique physiology or the unique effect of these small microbial secreted molecules, one can start to understand how some microbiomes that live in one place could impact health and disease processes that occur miles away, for example, gut microbes impacting the brain or the joints. And many of these effects could be mediated by these small bioactive molecules. In fact, we and others have measured the small molecule repertoire in peripheral bloods of both animals and humans, and it seems that around 50% of all small molecules that are found within our peripheral blood may originate in one way or another or be modulated in one way or another by our gut microbes. It's a big thing. It's a big story because it means that our microbes could be regarded as a neglected organ that has very distant effects that were not previously anticipated.
Dr. Patrick: I think most people that, you know, listen or watch our podcast are familiar with some of the beneficial metabolites that are produced like these short-chain fatty acids like butyrate, or propionate, acetate, and their effects on modulating the immune system. And I think there's been just overwhelming evidence at this point that there's a role in these short-chain fatty acids for, you know, playing signaling molecule role where they affect T regulatory cell activity and/or production, for example. What about the flip side of that about compounds that are produced by bacteria in our gut that are not beneficial and what role, for example, like leaky gut or, you know, what would be more technically intestinal permeability, some compounds that can be, you know, produced or this concept of metabolic endotoxemia, for example, maybe what role that could play in even cardiovascular disease risk?
Dr. Elinav: That's a great question. And it leads to an observation made by a clinician decades before we knew there was a microbiome or appreciated the potential magnitude of the effect of the microbiome on human health. And this relates to the ability of the gut to withstand the huge antigenic and foreign molecule burden that it sees every day in the form of food, and the trillions of microbes that are in the intestinal lumen are separated from our sterile self by only a single layer of intestinal epithelial cells. And throughout evolution, our human body has developed amazing means to kind of provide this protection from invasion of foreign molecules into our sterile body while preserving the ability of our intestines to absorb food or food molecules, which is totally critical for our existence.
What we observed for many decades was that this healthy leakiness of the gut that enables us to absorb food is disrupted in some disease context leading to an altered ability to withstand or to separate these foreign objects or these foreign molecules which now penetrate into the human body, and they ignite, they turn on the immune system in ways which lead to disease. And this leaky gut or this altered gut permeability as we call it seems to constitute a common denominator factor which is found in many disease states such as heart disease, many cancers, many autoimmune disorders. And for many years, we did not understand the precise mechanisms by which this leaky gut forms and what the consequences of this leakiness are on human health. In the last decade, there's been a lot of research focused on trying to understand this important concept.
What we've contributed was an understanding that diverse molecules that are secreted by gut microbes are critically important in determining the normal state of leakiness that allows us to absorb food on the one hand but blocks all the foreign molecules that we don't want in our body from entering the body under normal circumstances. And once the conditions arise that lead to the disruption of this normal barrier function, which leads to leakiness, then this leakiness results in the influx of molecules from the gut into the sterile human body, which contribute to disease states or to exacerbation of disease in different contexts. So it's just another important mechanism by which our microbes could lead to an increased disease susceptibility or to new severe symptoms in a previously present disease based on their effects on the gut barrier.
Dr. Patrick: What do you think the main contributing factor...environmental factors to, you know, this "leaky gut" or, you know, disrupting the balance, you know, of the gut barrier where you're basically...your immune cells are now sort of is having contact with the bacteria in the gut and it's causing this immune response? What are like a couple of the top main environmental factors that cause that?
Dr. Elinav: It's a great question. And we need to understand that this barrier which we refer to is also a very complex structure, and it is composed of the lining cells of the gut, the epithelial cells of the gut which are characterized by very specific connections to one another which are tightly regulated. And these tightly regulated connections between the cells could be influenced by molecules that come from food. They could influence by molecules that come from the microbes. And once this regulation is disrupted, then leaky gut occurs. A second part of the barrier is the mucus layer that overlays these lining epithelial cells, and the mucus layer in the gut is exceedingly important in separating the bacteria and the food molecules from physically attaching to our human side, to the epithelial cells. And it is increasingly shown that the generation and the preservation of this protective mucus is also regulated by a number of bacterial and environmental factors such as medications, toxins, and food components. So, the more we learn, the more we realize that these protection barrier-related processes are intriguingly impacted by the environment and by relays of the environment on our gut microbes.
Dr. Patrick: Do you think the food composition component is something that is, you know, more of an individualized...there's an individualized response to that that could affect the gut permeability, or is there some general phenomenon like, you know, too much sugar in combination with saturated fat, for example, you call this an obesogenic diet?
Dr. Elinav: I think that, you know, the more we look, the more personalized we see that the effects are. So just to give you an example of one food component that would impact some humans but not others. Let's talk about celiac disease, right, celiac disease mediated by proteins that are present in certain crops. For example, gliadin is the major protein that is a part of bread, what makes our dough sticky, and what makes our bread tasty. And in individuals that suffer from a genetic susceptibility to develop immune reactivity to this protein, then a cascade of immune reactions occurs that leads among many other changes to a leaky bowel or to a leaky gut which contributes to disease state in the celiac patients. So this is I think a nice example of host genetic risk factors combining with food components that these at-risk individuals are exposed to that lead to a clinical manifestation of disease, in this case, celiac. But I think this is just the tip of an iceberg, and many of the complex diseases that we call a multi-factorial diseases because we don't know what causes them such as heart disease and even maybe some cancers are caused by a combination of genetic risk factors coming from within the body and food and microbial components that contribute to a second hit, which leads to the clinical manifestations of these diseases.
Dr. Patrick: One could argue also that the lack of a dietary composition or lack of a type of food could also play a role as well, and I think a big one there would be, you know, these...what I'd like you to kind of dive into a little bit, but the fermentable fiber. There's the prebiotic, the probiotic, which most people are familiar with, and then there's the postbiotic. Can you describe the differences between these?
Dr. Elinav: Yes. So these are kind of terms that are aimed to simplify the different or some of the different interventions which are heavily researched in the young microbiome field in trying to modulate the microbiome and its interactions with the human body in trying to generate new treatment. So prebiotic interventions are classically defined as food-related interventions that are composed of dietary fibers that are aimed to "make our microbiome healthier," whatever that means. I think that our Personalized Nutrition approach is a more data-driven development of this prebiotic intervention. A probiotic intervention is one which involves the supplementation of exogenous microbes that we hope would be welcomed by our indigenous microbiome and impact our body in a favorable manner. And a postbiotic therapy is one which utilizes these small bioactive molecules which I've mentioned before, these metabolites, and once we understand whether a metabolite is missing in some disease context, now we can supplement this missing metabolite by a postbiotic therapy, thereby bypassing the entire microbiome or the entire microbial ecosystem, which is so difficult to intervene because of inter-individual variability. So that's postbiotic therapy. And on top of it, one could add other interventions such as the fecal microbiome transplantation, phage therapy, and other eradication therapies that are being developed and more interventions that are being explored.
Dr. Patrick: I do want to get into phage therapy in a moment, but before we go there, your lab has published some research that...or some data that has indicated or suggested that probiotics or supplementation with probiotics may work for some individuals but perhaps not others. What was the mechanism for that? Why is that?
Dr. Elinav: Yeah. So like anything or almost anything that we study especially in humans, we find that the indigenous microbiome in its distinct inter-individual uniqueness plays major unappreciated roles in determining many of our microbiome-dependent health outcomes. So when we studied probiotics or we studied 11 different types of commonly prescribed over-the-counter probiotics, and we studied them both in mice and in humans in probably the most invasive microbiome study performed to date, we found that in around half the people that we've tested, when they take these probiotic bacteria and supplement them into their diet, the probiotics are met with a very hostile microbiome, indigenous microbiome, which does not let them colonize our gut, even temporarily. So you take these probiotics in these cases, and they end up very rapidly from one end to the other. And by sampling these volunteer participants by invasive colonoscopy and endoscopy at different stages of probiotic exposure, we could find that in individuals that consume probiotics but are not able to colonize these exogenous bugs along their gut, we could see absolutely no impact on the gut responsiveness to these exogenous probiotics. However, in the other half of the individuals, the microbiome was much more welcoming, and when they were eating the probiotics, the probiotics at least temporarily were able to colonize along their guts. And in these individuals, we saw that these exogenous microbes indeed had quite significant impacts on our measurements of human responsiveness at least in the gut. That tells you that even the colonization of exogenous probiotics is highly individualized and is mainly determined by the composition and the function of the indigenous microbiome and how it welcomes these new microbes that come into the neighborhood.
Dr. Patrick: I have about three follow-up questions for you on this. So for one, is it known what exactly the...you know, what's regulating this, whether or not there's, you know, residential space or space made available for supplemental probiotics to colonize? Is it just the types of bacteria that are a little bit friendlier to say, "Yeah, you can come stay here"? Or are there other factors that also regulate whether or not there's any residential space available?
Dr. Elinav: This is a great question. And the more we answer, the more we realize that this interaction between different microbes, whether they're members of the indigenous microbiome or members of the microbiome meeting these exogenous probiotics... The nature of these interactions is highly complex and poorly understood and is probably composed of many different ways of interaction. Sometimes the microbes just compete for space or compete for food. So if one microbe is more adept in eating the food at the expense of another, then it would expand and would not let the other thrive. Another potential set of interactions are mediated by the secretion of what we call antimicrobial peptides, which are these types of natural antibiotics which some microbes are able to secrete which inhibit others. So you can see that some of these interactions are very hostile, while others could be very supportive in providing nutrients by one microbe that would enable the survival of another. It's a whole big zoo of interaction that we're just trying and starting to unravel.
But the one take-home message which we've already discovered, and this was quite shocking to us, was that both in the human and the mouse setting, when you disrupt the indigenous microbiome by the administration of antibiotics, for example, you kind of empty out the neighborhood, and now you give probiotics, now the neighborhood is empty and the probiotics are no longer met by resistance, and now they can colonize the gut. But the result of this colonization is not always positive, and what we've discovered was that in people who were given probiotics together with antibiotic administration, which is a very common practice around the world, in the U.S. for sure, the probiotics were now able to colonize the gut because the indigenous microbiome was at least temporarily eradicated by the antibiotics, but now these probiotics were very persistently inhibiting the return of the indigenous microbiome after antibiotic exposure was no longer present. In other words, by giving probiotics together with antibiotics, we may be protecting some individuals from the adverse effects associated with antibiotic treatment, but the price that we may pay is the creation of a chronic disturbance in the composition of our gut microbiome with the microbiomes...with the probiotics very aggressively refusing to leave the neighborhood and colonizing the once diverse gut and not letting the microbiome repopulate and recolonize. And this could have long-term effects in predisposing individuals to chronic diseases, which we and many others are researching. And this tells you that probiotics... We're not against probiotics. We're just very much in favor of studying them in a very comprehensive manner in order to make sure that we understand their functions, their personalized effects, and their possible long-term influences, whether they're good or bad, on human subjects.
Dr. Patrick: I guess there's a lot of questions that also do arise from that data, including, you know, the indigenous microbiome composition before the antibiotic treatment, for example. Perhaps it was not a good one. So then you wonder, "Well, maybe I don't want some of those bacteria to be, you know, inhabiting my gut again." Or perhaps, you know, the timing and the quantity of the probiotics. So maybe you shouldn't just be overwhelming your gut constantly with them, but maybe, you know, if you want just to seed a little bit of some of these bifidobacteria or some bacteria that may be beneficial. Is that something that you guys are looking into or thought about?
Dr. Elinav: I totally agree. I don't think that these results per se, you know, tell you anything definitive that is a take-home message. It tells you that we need to be careful until we know better. But it also tells you that potentially, you know, if you could combine antibiotics with probiotics in a diseased microbiome setting, then they could potentially be beneficial, you know, could get rid of a disease associated or a disease-causative microbiome, replace them with probiotics to keep the niche occupied, and then maybe you'll do something good to diseases.
All I'm saying is that we need research and we need evidence. I oppose, you know, careless manufacturing of probiotics just because they don't impact the taste of food. And our...and other people's research suggests that what we call precision probiotics or next-generation probiotics may be bugs. They don't smell the best, maybe they're not the tastiest bugs, but they may be most effective in colonizing the human gut and positively impact our health in different contexts. We just need to understand how they do it to tailor them to the individual and to make sure that they're safe.
Dr. Patrick: Two questions to follow up on that. Do you think that, one, the amount or the dose of probiotic or the colony forming units, for example, can play a role in whether or not a probiotic can colonize if there's any at all? And two, do you think that these probiotics...? Perhaps they're not colonizing, but they're exerting a therapeutic effect as they are flowing through the gut and particularly in people with a disease like colitis or inflammatory bowel disease or something obviously that would be a disease that originates in the gut. Do you think there would be a benefit even if the probiotic is not colonizing but just the fact that you are flow through and it's helping a person with some gut issues?
Dr. Elinav: That's a good question. And I can tell you that our...at least in the strains that we've tested, we've given the volunteer participants quite heavy doses of these probiotics. So when they were not colonizing...they were not colonizing. And we are the first to study this colonization pattern, not in stool, which is where most of the previous studies have looked into in probiotics. We found that the stool is very problematic in assessing colonization because, even in people who do not colonize at all with probiotics, you know, they end up accumulating in stool because that's the natural way where they go. So you need to really sample inside the gut in order to understand whether a person colonizes or not. So in the people who did not colonize even when we gave them high doses of these preparations, we could observe absolutely no colonization even when assessing it by very sensitive means along the gastrointestinal tract. Now, whether they could have some effect when they're, you know, flying through the lumen all the way to where they end up, putatively maybe but in my view, very unlikely. You're talking about bugs that secrete molecules that are kind of dispersed in an ocean, you know, they're diluted in an ocean. And to think that they would be physiologically effective when in the middle of the ocean, you know, is at least theoretically possible. But I think the burden of proof is on those who claim it.
My hunch or my assumption is that most of the effect that one would see from exogenous bacteria, for example, from precision probiotics would be expected to exert along the mucosal surfaces where the microbes meet the epithelial cells, where they adhere to the epithelial cells or to the mucus layer, and where the distances are such that secreted molecules could reach their destination without having to pass through this giant ocean.
Dr. Patrick: Yeah. I mean, that definitely makes a lot of sense. Perhaps there's a role for dose in the bacteria having any sort of therapeutic effect as flow through. I've personally, you know, read quite a few studies on a certain very high-dose probiotic over 400 billion bacteria. Many of the studies at the time, the bacteria was...the brand was called VSL#3. The formulation was like done again, and it was called Visbiome. But many of the publications...clinical studies, you know, including as well as animal studies, but there have been benefits, for example, on like colitis or irritable bowel syndrome with taking either 400 or 800, you know, colony-forming units, so like, you know, 800 billion, so much higher doses than you would find in something on the shelf of a grocery store. And again, it might just be, you know, a very transient effect that's happening, you know, preventing diarrhea, for example, when you're taking a high dose, probiotic or something like that.
Dr. Elinav: I cannot rule it out, but I respectfully disagree that the level of evidence is even close to one which would make me recommend these for IBD or for any disease. And the evidence is that not a single probiotic preparation to date has been approved as a medical intervention by the FDA or by the European counterparts of the FDA. So, again, I'm not against probiotics, but I think that just like any human medical intervention, probiotics should be assessed by evidence-based medicine and proven to be effective in certain preparations, certain doses, certain medical conditions, and not assumed to be effective before we test them and before we prove them.
Dr. Patrick: Well, you mentioned something earlier about the combination of perhaps even probiotics with bacteriophage or combination of antibiotics with bacteriophage. Can you explain to people what bacteriophages are?
Dr. Elinav: Absolutely. So bacteriophages are intriguing viruses that in contrast to the viruses that we all, you know, will suffer from these days are viruses that do not infect humans, and they do not infect any mammals or any eukaryotic cells. These are viruses that only infect bacteria and only attack bacteria. And these viruses are exceedingly common in nature. You can find many, many types of these viruses in our environment. And in fact, these viruses are the big enemies of the bacteria that surround us. So there's kind of an arms race between bacteria and these bacteriophages which attack bacteria, with the phages trying to kill bacteria and the bacteria developing a means of defending themselves against these viruses. It's an intriguing arms race which led to some groundbreaking discoveries such as CRISPR, which is, you know, one of these defense mechanisms which has been now massively exploited by science in order to genome edit, for example, genes of interest. This is what occurs in nature.
What we and others are thinking about in utilizing phages is that, you know, we have a huge unmet need in the microbiome field. Imagine that you find a member...a microbe, a bacteria in the microbiome which contributes to disease, contributes to IBD, contributes to cancer. What do you do? How do you get rid of these bacteria without harming the entire microbial surrounding that is so important for our health? Antibiotics are very limited manner of doing this. Antibiotics are non-specific. They have big adverse effects. They result in the emergence of resistant strains. So you cannot use antibiotics forever for your entire life. And many of the disease-causing bacteria in the microbiome are antibiotic-resistant. So what do you do? We have really an unmet need in having no means of taking out a microbe from the microbiome when we want to eliminate its bad effect.
And so we thought that phages could represent an attractive means of attacking a bacteria without impacting the entire microbiome because phages are very specific in their targets. A given phage would only attack a certain family of bacteria that has receptors which the phage recognizes. Now, I told you before that bacteria as part of this arms race have developed very strong defense mechanisms against phages. So if you give just one phage, it is very likely that the bacteria it would attack would generate defense mechanisms that would make it resistant against this phage, and so your therapy would not be successful.
So what we are doing, we are generating cocktails of phages that are targeting the same bacteria through different receptors or different mechanisms, and together these phages are killing the bacteria without allowing it to develop this antiphage defense system. And if this is successful and we're now in the midst of clinical trials, we would be able to really take a needle out of the haystack by targeting a single bacteria or a single type of bacteria without killing the entire microbiome and causing a substantial collateral damage.
Dr. Patrick: I wasn't aware that the bacteria were developing these defense mechanisms kind of you can think about it, you know, as similar to like antibiotic resistance in a way, I guess. What are your thoughts about then perhaps a future where we have this targeted type of treatment where in addition to maybe your bacteriophage cocktail that's targeting maybe, you know, one or perhaps two of the pathogenic type of bacteria and then combining them with a commensal type of bacteria in terms of, you know, allowing these precision probiotics in a way, perhaps, I don't know, maybe there's another name for it, but where you're actually allowing the types of bacteria that we know are commensal that maybe perhaps these people are not...are lacking and this is a way to actually get them to be colonized?
Dr. Elinav: Absolutely. I totally agree with what you suggest. And I think, you know, at the very young microbiome field, we're at the stage of understanding more and more of these interactions and the roles of different bugs and their communication systems, and also are increasingly busy in generating these new treatment options that hopefully would be put on the clinical shelf in years to come. But I am totally with you with the prospect that these new interventions would be combined with each other in contributing to what we call personalized or precision medicine. In other words, I would speculate that exactly as this suggests, a phage cocktail that would eradicate a family of bacteria from the microbiome would be successfully combined with a probiotic or maybe a precision or next-generation probiotic which would have the ability to colonize in a given person and would replace the niche now freed from this disease contributing microbe. So a combination between probiotics and phages between dietary interventions that would enable better probiotic activity and so on and so forth are what I anticipate for our future.
Dr. Patrick: What do you think the potential timeline would be on this, you know, ultimately replacing some of our current ways of like antibiotic treatment, for example, which is a very blunt sort of...you know, it uses a very blunt mechanism? As you mentioned, it wipes out everything, good and bad bacteria.
Dr. Elinav: Yeah. I mean, when we criticize antibiotics, we need to be very careful. You know, antibiotic interventions have amazingly transformed human lives, human health, human medicine. You know, they increased I think close to 30 years of life span within a century and at least partially took care of what is considered to be our number one, two, and three cause of mortality for millions of years. However, as we discussed previously, antibiotics are also associated with many prices that we pay, and we're just beginning to appreciate what they do to our microbiome. I don't think that the current medical interventions would be replaced, but I am very hopeful that we would be able to implement them with new precision data-driven approaches that would enable to increase the efficacy of these treatments and to be combined with them. As to the timelines...you know, there is quite a hype or overhype with the very young microbiome field, and partially, it's justifiable because, you know, in a matter of a decade and a half, we've discovered that our human body in addition to the 20 something thousand genes that are encoded in our human cells also contained 3 million and more bacterial genes that we didn't appreciate and we didn't know anything about. So this is for sure at least in my view a revolution, but we're only at the beginning of understanding this new world.
Remember that, you know, it took decades for cardiology to get to a point where, you know, catheterization and all the fancy interventions that are saving lives today have been matured and developed for clinical use. We're only talking about a very infant field, lots of research, lots of advances, but also lots of challenges. I don't want to give a time estimate, but I'm hopeful that within the next decade, we will start to see some interventions maturing in a data-driven way into the clinical shelf.
Dr. Patrick: That would be great. What role do you think or what role does the so-called virum play in human health? And do you think that science may yet find that viruses modulate health like in unexpected ways?
Dr. Elinav: Absolutely. And I think one of...the only reason we're so much into bacteria in the microbiome especially in the gut microbiome is because we have the tools, and we're a little bit lazy in kind of searching "under the lamp" and going where it's comfortable. But the more we probe into the virome and the fungome and the parasitome, we find that there are whole kingdoms within our microbiome which are understudied and underappreciated, but nonetheless, I think that they have potential huge impacts on how the human body behaves in health and on the risk of developing disease and even on other kingdoms within the microbiome. So there is huge amount of exciting research to be conducted in decoding these roles of these other kingdoms, and, you know, the future will tell us.
Dr. Patrick: Well, it's very, very exciting. And I kind of just want to follow up. I do want to ask you about some of the top lifestyle modifications. And I know that we've been talking a lot about personalized nutrition, so it's challenging to answer that question. But before we get there, just out of my own interest, we've talked a lot about the compounds that are generated in the gut perhaps from, you know, many of the bacteria in the gut and how these compounds can have beneficial effects on human health and also can have detrimental effects on human health. And there's one compound that I've been following for a while and I continue to follow, and it's a compound that is associated with atherosclerosis and heart disease. It's TMAO and it's produced from precursors like L-carnitine or even choline which are found in red meat and eggs respectively. You'll find a lot of conflicting evidence looking at, for example, the observational data, epidemiological studies where you see, you know, people that eat red meat and/or, you know, eggs. If they are healthy and they don't have metabolic disease, they don't have type 2 diabetes or dyslipidemia, they don't have unhealthy lifestyle factors, so, for example, they're active, they don't smoke, they don't excessively drink, they're, you know, not overweight that they actually don't have a higher cardiovascular disease risk or mortality or all-cause mortality as people that are not consuming those types of foods that are high in L- carnitine or choline. But you'll see if people have unhealthy lifestyle factors, they do have an elevated risk. You'll see a lot of conflicting evidence, and it's you're trying to figure out, "What do I eat? What do I not eat?" What role does the microbiome play in the production of the TMAO which is thought to be associated with heart disease? Yeah.
Dr. Elinav: It's a great question based on a great set of stories by Stan Hazen's group, which I think contributed a very important concept to our understanding of how the microbiome cooperates with the human body in generating together compounds which may impact human health. So in this particular case, we're talking about a connection between dietary compounds such as choline and carnitine, which are digested by the microbes into a compound called TMA, which then influxes into the host and is further converted by the host...by the liver of the host into TMAO, and this TMAO swims into the circulation where in some instances, it could impact macrophages that form plaques that are responsible for atherosclerosis and it's potentially devastating health effects, heart disease, brain disease, kidney disease, and more. So from a fundamental microbiome perspective, this is a fine example of a cooperation that exists between dietary cues that are perceived by the microbes and then further modulation by the host that leads to a health outcome.
Now, you're absolutely right that if you look at a health perspective, and now I'm speaking as a physician, you know, you cannot explain atherosclerosis by just one factor. You cannot say that, you know, one type of microbial reaction or one type of food or even one genetic risk factor in a human individual would explain the entire spectrum of this huge and highly variable disease. By definition, these common multifactorial diseases are influenced by a combinatorial collection of risk factors. And I think what this fascinating study has provided was the proof of concept on how mechanistically one could explain the influences of particular types of diet and the microbes on the risk of developing a particular disease in some individuals with other risk factors that contribute to this disease. So I would never expect that every individual that would be exposed to the same levels of carnitine or would feature the same bugs that convert choline into TMA would develop heart disease. It's a combination by many different risk factors.
Coincidentally, we've recently published another study focusing on a peculiar type of obesity that develops after cessation of cigarette smoking, and to make a long story short, we found a similar cooperation between the microbiome and the host in generating compounds that could drive this obesity phenomenon after smoking cessation. So it seems that the concept which we termed the "holobiont" concept, in which you can regard a human as a combined set of microbes and human cells, could contribute to many of the more complex health outcomes that are so concerning to many of us.
Dr. Patrick: Well, with that said...this has been a really interesting conversation, Eran. Thank you so much. We've talked a lot about precision medicine, personalized nutrition, and how people respond differently to foods. So it's a little hard to, you know, come up with a top lifestyle modifications or, you know, to improve gut health. But, you know, in your opinion, are there some low-hanging fruit? We are not there in terms of our precision medicine and personalized nutrition yet. We're beginning to understand a lot more about it, thanks to research from your lab and others. But are there some low-hanging fruit, things that like, you know, maybe perhaps consuming foods that have some of these fermentable fibers or prebiotics like you mentioned or fermented foods that also have probiotics and things like that?
Dr. Elinav: It's a great question and a question that I'm being asked very often. I can tell you that what we've been discovering in our own studies, even, you know, without looking into the personalization aspect, is that some of the behaviors, you know, which your grandmothers would recommend to you are also beneficial in terms of what they do to the microbiome. So, for example, maintaining healthy sleep patterns and avoiding as much as possible erratic sleep weight behavior has very profound effects on our measurement of the microbiome, how it impacts our regulation of weight and the glucose or sugar metabolism or the avoidance of type 2 diabetes, for example. In terms of fiber, you know, in general, I think that the data is quite solid in promoting fiber as a good, you know, family of foods to consume. However, I must say that we and others are engaging very exciting studies, which suggests that even with fibers...not all fibers are created equal. In other words, you know, even fibers are composed of many different chemical formulations that differ from each other in the way that they are consumed by the microbes and impact the human body. So even with fibers and with the generally beneficial effects that have been observed with them, it seems that some fibers are better than others. And we're trying to contribute towards new knowledge that would refine this recommendation in different individuals and with different fibers. You know, smoking seems to be a universally bad behavior for many reasons, but when we measure what it does to the microbiome, we were intrigued to find that many cigarette-related chemicals not only reach the systemic circulation, but they actually penetrate the gut, and they impact the microbiome towards a disturbed composition and function. And this has its own independent effects on, for example, the risk of developing obesity after you start...you attempt to stop smoking. So all of these behaviors, which in many cases we know are probably not good for us, are also not good for us in terms of their effects on the microbiome. Beyond this, I think that we need data, we need knowledge, we need to increasingly learn to harness diet to the individual in order to really optimize the power of the microbiome in impacting human health.
Dr. Patrick: And what about the timing of our food intake? Would you say that's a pretty top...?
Dr. Elinav: I can tell you that in our personalized nutrition machine learning algorithms, which are used to predict a person's dietary responses in a very accurate manner, the timing of our diet and even the timing of our meal last night are part of the features that are used by this unbiased algorithm in order to form its very accurate predictions. In other words, it seems that the timing of our diet is important for many different aspects coming from many different studies by us and by others. What we do with it in addition to, you know, trying to time our diet in a kind of normal and routine manner is still under review or under research.
Dr. Patrick: This algorithm that you were just referring to. So this is a top company that was... Was it started by you or...?
Dr. Elinav: Yes. So basically, the Personalized Nutrition Project was an ambitious project which was headed by me and my colleague, Eran Segal, who's a mathematician from the Weizmann Institute of Science. We started with this project back in 2012, and this was a study that was first published in 2015 and formed the cornerstone of what we call personalized nutrition today. And in this study, we analyzed the data from 1,000 individuals in Israel that kindly gave us the week of their life, and we measured and collected an unprecedented amount of microbiome and host-related data, including a smartphone app that was used in this study, and a continuous glucose measurements that generated very accurate measurements of sugar responses to food in a week of follow-up. And then a very sophisticated machine learning and AI technologies were used to generate predictive algorithms for each individual that are able to accurately predict a person's sugar responses to any given food. And this eureka moment was the basis for personalized nutrition because it allowed us for the first time to formulate diets that are different between individuals but would hopefully lead to normalization of blood sugar levels. And this was tested by us in different contexts including recently in a long-term randomized human trial which compared this data-driven personalized approach to the gold standard American Diabetes Association recommended diet. And we've quite convincingly shown that this personalized science-driven approach was outperforming the current one-size-fits-all diet in a large group of pre-diabetic individuals, which are individuals already predisposed to develop disturbances leading to type 2 diabetes. This set of discoveries has been repeated by other groups across the world and is gaining track and basically tells us that data coming from the host, from the human host and data coming from the microbiome could be combined using advanced technologies in order to predict and maybe to impact dietary interventions at different clinical contexts.
Dr. Patrick: So this company...I know it's called DayTwo. Does a person have to wear a continuous glucose monitor or like, you know, there's a bunch of biomarkers that need to be done to try this out with your...?
Dr. Elinav: That's a great question. And just to make clear to our audience, all the research that I've stated was academically done in an academic setting without any company involved. But following the publication, the Weizmann Institute of Science have licensed the technology to a spin-off company called DayTwo, which further developed it for massive use in app scaling by many more individuals. What the advantage is of DayTwo as a company is that now that they've performed over 100,000 tests on 100,000 people and more, the quality of data that was collected is so great and the resolution is so great that the people that are now engaged no longer need to go through all the procedures that characterized the early studies, and they don't even need to wear our continuous glucose monitor anymore. In other words, a person can now provide a stool sample that can be shipped through the mail plus some commonly available clinical parameters that they can provide through the internet. And then an accurate prediction of that person's glycemic responses or sugar responses to foods and recommendations that are peculiar and specific for that person could be provided because of the background database that was already created.
Now, I'm not part of the company. I'm one of the two scientific founders of the company, but the company is now running on its own mainly in the U.S. and is available in the U.S. The findings that we discovered have been reproduced by others in other human studies in the UK and in the U.S. There are other commercial entities that are developing the same approaches. I can tell you that in the book we published called "The Personalized Diet" in addition to our story, we also describe kind of a do-it-yourself, a non-commercial way to exploit these discoveries. For example, by buying a glucose monitor that, you know, you can purchase in your local pharmacy, and by skin pricking yourself and measuring your blood sugar responses after some of the foods that you usually consume at your daily lives, you can now start to tweak your diet and to change ingredients in your diet in reducing your sugar responses after meals. So you can do it yourself in, you know, of course, much less sophisticated manner, but you can use the same principles that we've discovered in changing elements in your diet and making your sugar responses lower than before.
Dr. Patrick: So it sounds like you're a proponent of people wearing a continuous glucose monitor. I've worn one for the past...oh, almost three years, and I have learned an immense amount of very interesting information from wearing one, probably one of the most surprising ones early on. I started wearing it when I was a new mother and was the effect of lack of sleep on how the way my body responded to the same foods that I've always eaten in terms of my postprandial glucose response, and it was completely out of control when I was...when my sleep was disrupted, you know. There are scientists and researchers out there and physicians that do not like the continuous monitor, you know, glucose-wearing approach because they claim that it, you know, urges people to not eat a healthy fruit or something like that because it may elevate their blood glucose level. What do you think in response to that?
Dr. Elinav: I'm not sure I would like to propose that every person wears a continuous glucose monitor. But I respectfully disagree with those who say that, you know, measuring yourself or using science and technology in order to improve, you know, what you do in your daily lives would, you know, be wrong. I think that, you know, disregarding all the advances that science is proposing to us and not utilizing these advances for our benefit would probably make us miss a lot of the good that science has to offer. You know, by wearing a continuous glucose monitor, you probably experienced many surprises. And maybe, you know, we've done thousands of people, and I can tell you that almost in any person that we've measured, we found counterintuitive surprises. Some people spike their blood sugar to the roof when they eat tomatoes. Now you combine tomatoes with some white bread and the response goes down. So, you know, by not doing the experiment or by not measuring themselves, they would devoid themselves from the benefits of knowing what is good and what is less good for themselves. So I'm all for measurement. I'm all for knowing and for doing this rationally and carefully but doing it.
Dr. Patrick: I'm 100% in agreement with you. So your book is "The Personalized Diet." You co-wrote it with your collaborator Dr. Eran Segal.
Dr. Elinav: Segal.
Dr. Patrick: Segal?
Dr. Elinav: Yes.
Dr. Patrick: Okay. And DayTwo, which is now licensed by the Weizmann Institute for Science, is the app you were talking about. But again, thank you for talking about the alternative approach with perhaps even people getting a continuous glucose monitor. If people want to follow you, you're on Twitter. Your Twitter handle is elinav_lab. So that would be E-L-I-N-A-V, underscore lab, L-A-B. And you also have two lab websites. If you google your name Elinav...yes. Sorry, E-L-I-N-A-V, you'll find all the lab research that you're doing. Phenomenal. I mean, an amazing impact that your research has had on our understanding of the interaction between the microbiome in our gut and human health. And I'm so happy that we were able to connect and have a conversation today. I've been a big fan of your research for a long time now. So thank you so much for coming on the podcast and taking time to have this very interesting discussion with me.
Dr. Elinav: It's my absolute pleasure, and great talking to you, Rhonda.
A type of time-restricted dietary pattern. When practicing 16:8 intermittent fasting, a person eats all their meals in an eight-hour window of time (typically during the day) and abstains from food for the remaining 16 hours. Evidence suggests that 16:8 intermittent fasting is beneficial for managing weight and improving metabolic function in people with obesity.[1]
- ^ Chung, Hye Soo; Cho, Sung Tae; Kim, Tae; Oh, Chang-Myung; Moon, Shinje; Kang, Jiseung, et al. (2020). Beneficial Effects Of Time-Restricted Eating On Metabolic Diseases: A Systemic Review And Meta-Analysis Nutrients 12, 5.
A short-chain fatty acid produced by microbes in the human gut. Microbial production of acetate occurs primarily during the fermentation of indigestible fibers in the colon. Acetate may be beneficial in maintaining body weight and healthy glucose metabolism.[1]
- ^ Canfora, Emanuel; Hernández,; Jocken,; Blaak, (2019). The Short-Chain Fatty Acid Acetate In Body Weight Control And Insulin Sensitivity Nutrients 11, 8.
The body's fat-storing cells. Also known as adipocytes or fat cells, adipose cells store energy in the form of triglycerides. Adipose cells are hormonally active and play important roles in metabolism and physiology.
A diverse class of molecules also known as host defense peptides. Antimicrobial peptides are produced by the immune system of many multicellular organisms and by microorganisms in the human gut. They exert broad-spectrum specificity and low toxicity against pathogens and work either directly or indirectly, via modulation of endogenous immune capabilities. Antimicrobial peptides show promise as potent alternatives to current antibiotic therapies.[1]
- ^ Naas, Thierry; Sabatier, Jean-Marc; Bechinger, Burkhard; Rima, Mohamad; Fajloun, Ziad; Rima, Mariam (2021). Antimicrobial Peptides: A Potent Alternative To Antibiotics Antibiotics 10, 9.
A bioactive flavonoid compound present in many fruits and vegetables, especially parsley, onions, chamomile, and certain citrus fruits. Apigenin exerts potent antioxidant, anti-inflammatory, and anticancer properties. Recent evidence indicates that apigenin plays important roles in metabolic health via its influence on thermogenesis.[1]
- ^ Castillo-Quan JI (2012). From white to brown fat through the PGC-1α-dependent myokine irisin: implications for diabetes and obesity. Dis Model Mech 5, 3.
Sugar substitutes that provide few or no calories. Artificial/non-nutritive sweeteners are derived from a variety of natural and synthetic sources. Current FDA-approved artificial/non-nutritive sweeteners include saccharin, aspartame, acesulfame potassium (Ace-K), sucralose, neotame, advantame, stevia, and monk fruit (also known as luo han guo) extract. Some evidence suggests that consuming non-nutritive sweeteners induces gut microbiota dysbiosis and promotes glucose intolerance in healthy people, potentially driving the development of type 2 diabetes.[1] However, the overall uniformity or consistency of this effect across different types of sweeteners remains unclear, with some sweeteners having purported beneficial health effects in some contexts.[2] [3]
- ^ Szewczuk, Myron R; Qorri, Bessi; Liauchonak, Iryna; Dawoud, Fady; Riat, Yatin (2019). Non-Nutritive Sweeteners And Their Implications On The Development Of Metabolic Syndrome Nutrients 11, 3.
- ^ Suzuki, Yasushi A.; Tomoda, Mayuko; Murata, Yuji; Inui, Hiroshi; Sugiura, Masaki; Nakano, Yoshihisa (2007). Antidiabetic Effect Of Long-Term Supplementation With Siraitia Grosvenori On The Spontaneously Diabetic Goto–Kakizaki Rat The British Journal Of Nutrition 97, 4.
- ^ Mathew, Justin; Kuzmar, Isaac; Angarita Dávila, Lisse; Rojas, Edward; Bermúdez, Valmore; Motlaghzadeh, Yasaman, et al. (2018). Stevia Rebaudiana Bertoni And Its Effects In Human Disease: Emphasizing Its Role In Inflammation, Atherosclerosis And Metabolic Syndrome Current Nutrition Reports 7, 3.
An immune disorder characterized by an immune response to and subsequent destruction of the body’s own tissue. The causes of autoimmune diseases are not known, but a growing body of evidence suggests they may be due to interactions between genetic and environmental factors. Autoimmune diseases affect approximately 7 percent of the population in the United States and are more common in women than in men. Examples include type 1 diabetes, Hashimoto’s thyroiditis, lupus, and multiple sclerosis.
A type of virus that infects bacteria. Bacteriophages are species-specific and typically only infect a single bacterial species or even specific strains within a species. For this reason, some scientists have posited that bacteriophage therapy may be a viable alternative to traditional antibiotics for the treatment of bacterial infections.[1]
- ^ Principi, Nicola; Silvestri, Ettore; Esposito, Susanna (2019). Advantages And Limitations Of Bacteriophages For The Treatment Of Bacterial Infections Frontiers In Pharmacology 10, .
A genus of bacteria known to inhabit the human gut. Bifidobacteria are anaerobic commensal bacteria. They are among the first bacteria to colonize the infant gut and may play critical roles in gut-mediated immune function.
Exerting an effect or effects on a biological organism, tissue, or cell. Drugs, vitamins, minerals, and many plant-based dietary compounds demonstrate bioactivity in humans, many of which provide protection against a range of diseases and health conditions.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A short-chain fatty acid produced by microbes in the gut. Microbial production of butyrate occurs in the colon during the fermentation of indigestible fibers, principally those from legumes, fruits, nuts, cereals, and whole grains. Butyrate exerts potent anticancer properties via its epigenetic actions on genes involved in colon cancer.[1]
- ^ Pradhan, Nibedita; Kar, Swayamsiddha; Parbin, Sabnam; Sengupta, Dipta; Deb, Moonmoon; Das, Laxmidhar, et al. (2019). Epigenetic Dietary Interventions For Prevention Of Cancer Epigenetics Of Cancer Prevention , .
A highly restrictive diet that includes only animal-based foods, including meat, fish, eggs, and some dairy products. Carnivore diets typically produce a ketogenic effect but may lack certain essential nutrients.[1]
- ^ O’Hearn, Amber (2020). Can A Carnivore Diet Provide All Essential Nutrients? Current Opinion In Endocrinology, Diabetes And Obesity 27, 5.
An autoimmune disorder caused by ingestion of gluten in genetically susceptible people. Celiac disease damages the absorptive lining of the small intestine, causing bloating, gas, pain, and diarrhea, while promoting weight loss, nutrient deficiencies, and other health disorders. The only treatment for celiac disease is strict adherence to a gluten-free diet.
An essential nutrient involved in a wide range of physiological functions, including neurotransmission, lipid metabolism, and cell membrane composition and repair. Humans can produce some choline in the liver, but most people need to consume choline in the diet to prevent deficiency. Dietary sources of choline include meat, eggs, fish, nuts, and cruciferous vegetables, among others. Evidence suggests that prenatal choline supplementation improves attention span in children.[1]
- ^ Bahnfleth CL; Strupp BJ; Caudill MA; Canfield RL (2022). Prenatal choline supplementation improves child sustained attention: A 7-year follow-up of a randomized controlled feeding trial. FASEB J 36, 1.
The body’s 24-hour cycles of biological, hormonal, and behavioral patterns. Circadian rhythms modulate a wide array of physiological processes, including the body’s production of hormones that regulate sleep, hunger, metabolism, and others, ultimately influencing body weight, performance, and susceptibility to disease. As much as 80 percent of gene expression in mammals is under circadian control, including genes in the brain, liver, and muscle.[1] Consequently, circadian rhythmicity may have profound implications for human healthspan.
- ^ Dkhissi-Benyahya, Ouria; Chang, Max; Mure, Ludovic S; Benegiamo, Giorgia; Panda, Satchidananda; Le, Hiep D., et al. (2018). Diurnal Transcriptome Atlas Of A Primate Across Major Neural And Peripheral Tissues Science 359, 6381.
A medical procedure that allows examination of the colon (large intestine). During a colonoscopy, a small camera is inserted into the rectum and colon to search for polyps or cancer or collect bacterial samples for analysis.
A measure of viable microorganisms in a sample that can multiply and form a community. CFUs are critical aspects of probiotic supplements, which typically contain 1 to 10 billion CFUs per dose, with some containing up to 50 billion CFUs or more. Higher CFU counts are not necessarily indicative of a probiotic's effectiveness.
A wearable health-monitoring device that measures blood glucose levels in "real-time." Originally designed for people who have diabetes, CGMs demonstrate tremendous value as diagnostic tools, providing a more accurate reflection of glycemic state by directly measuring the postprandial glycemic response to every meal and thereby highlighting individual differences in those responses.
A genome editing technology. CRISPR (short for clustered regularly interspaced short palindromic repeats) exploits naturally occurring gene editing processes used by bacteria. It targets specific stretches of genetic code and edits the DNA at precise locations, using the cell's own DNA repair machinery to add or delete pieces of genetic material. CRISPR can also be used to make changes to the DNA by replacing an existing segment with a customized DNA sequence. CRISPR may be beneficial in treating a wide range of genetic disorders, cancer, and other health conditions.[1]
- ^ Barrangou, Rodolphe; Doudna, Jennifer A (2016). Applications Of CRISPR Technologies In Research And Beyond Nature Biotechnology 34, 9.
Animals characterized by higher activity during the day and sleeping more at night.
A type of cell that forms the endothelium, the thin layer that lines the blood and lymphatic vessels. Endothelial cells regulate blood fluidity and fibrinolysis, vascular tone, angiogenesis, monocyte/leukocyte adhesion, and platelet aggregation. They are critical players in the body's immune response and resolution of inflammation.[1]
- ^ Kadl, Alexandra; Leitinger, Norbert (2005). The Role Of Endothelial Cells In The Resolution Of Acute Inflammation Antioxidants & Redox Signaling 7, 11-12.
Presence in the blood of endotoxin, which, if derived from gram-negative rod-shaped bacteria may cause shock.
An investigation of the distribution and causes of disease in a given population. Epidemiological studies are typically observational and include cohort, case-control, and cross-sectional studies.
A type of dietary fiber that undergoes fermentation in the gut, thereby promoting the growth of beneficial bacteria. Fermentable fibers are typically soluble and are found in grains, nuts, seeds, legumes, and some vegetables and fruits. Evidence suggests that fermentable fibers enhance gut barrier function and reduce inflammation.
Foods or beverages produced via the process of controlled microbial growth. Examples of fermented foods and beverages include yogurt, kimchi, sauerkraut, kombucha, and kefir. Evidence suggests that fermented foods and beverages may confer health benefits when consumed by humans. [1]
- ^ Whelan, Kevin; Dimidi, Eirini; Cox, Selina Rose; Rossi, Megan (2019). Fermented Foods: Definitions And Characteristics, Impact On The Gut Microbiota And Effects On Gastrointestinal Health And Disease Nutrients 11, 8.
Mice that lack all microorganisms. Germ-free mice are born and live in sterile conditions and serve as useful animal models in scientific research.
Glycemic response: The change in blood glucose concentration following consumption of a carbohydrate-containing food or beverage. Glycemic response is highly individualized and is a critical component of metabolic health. Evidence suggests that the microbiome participates in the regulation of host glycemic responses, ultimately influencing multiple aspects of human health.[1]
- ^ Suez, Jotham; Shapiro, Hagit; Elinav, Eran (2016). Role Of The Microbiome In The Normal And Aberrant Glycemic Response Clinical Nutrition Experimental 6, .
A concept that regards humans as complex meta-organisms composed of both microbial organisms and human cells. According to this concept, the holobiont regulates multiple aspects of human physiology, including metabolic, immune, and neurological function. The sophisticated web of interactions that arise from the holobiont likely contribute to myriad complex health outcomes.[1][2]
- ^ Levy, Maayan; Thaiss, Christoph A.; Elinav, Eran (2016). Metabolites: Messengers Between The Microbiota And The Immune System Genes & Development 30, 14.
- ^ Azcarate-Peril, M. Andrea (2019). Conclusions: What Is Next For The Healthy Human-Microbe “Holobiome”? How Fermented Foods Feed A Healthy Gut Microbiota , .
Complex, indigestible sugars present in human breast milk. The primary role of HMOs is to serve as prebiotics in the infant's gut. In turn, these beneficial bacteria produce short-chain fatty acids and other substances that prevent the colonization of pathogenic bacteria in the gut. More than 200 HMOs have been identified, and they are the third most abundant factor in breast milk after fat and lactose, averaging 20 to 25 grams per liter in colostrum and 5 to 20 grams per liter in mature milk. The quantity and composition of the HMOs in breastmilk are genetically determined and differ slightly between women.
An umbrella term for two chronic inflammatory conditions that affect the digestive tract – ulcerative colitis and Crohn's disease. Symptoms of IBD include diarrhea, rectal bleeding, abdominal pain, fatigue, and weight loss. Evidence indicates that dysbiosis, an imbalance in the types and numbers of microbes in the gut, contributes to IBD.[1][1]
- ^ a b Abdulkhakov, S R; Grigoryeva, Tatiana; Markelova, Maria; Danilova, N A; Vasilyev, I Yu; Boulygina, E A, et al. (2019). Markers Of Dysbiosis In Patients With Ulcerative Colitis And Crohn's Disease Terapevticheskii Arkhiv 91, 4.
A measure of how sensitive the body's tissues are to the effects of insulin. Insulin sensitivity defines a relationship between insulin production and glucose uptake. Poor insulin sensitivity promotes increased pancreatic insulin production, which can lead to increased risk for high blood pressure, heart disease and heart failure, obesity, osteoporosis, and even cancer.
Flavonoid compounds that exert bioactivity in humans. Isoflavonoids are found in legumes, such as soybeans, peanuts, and chickpeas, as well as other fruits and vegetables. Examples include daidzein and genistein (found in soy products) and formononetin and biochanin A (found in red clover). Evidence suggests that isoflavonoids exhibit antioxidant, antimicrobial, antimutagenic, antiproliferative, and anticancer effects.[1]
- ^ Miadoková E (2009). Isoflavonoids - an overview of their biological activities and potential health benefits. Interdiscip Toxicol 2, 4.
A byproduct of lysine metabolism. L-carnitine participates in several aspects of metabolism, including the transport of long-chain fatty acids into the mitochondrial matrix and regulation of pyruvate dehydrogenase activity. It also regulates pathways involved in breaking down and building up muscle. Evidence suggests that L-carnitine exerts antioxidant and anti-inflammatory properties, thereby attenuating exercise-induced muscle damage.[1]
- ^ Ringseis, Robert; Keller, Janine; Eder, Klaus (2013). Mechanisms Underlying The Anti-Wasting Effect Of L-Carnitine Supplementation Under Pathologic Conditions: Evidence From Experimental And Clinical Studies European Journal Of Nutrition 52, 5.
A type of lipoprotein. LDL is formed in the liver and transports lipid molecules to the cells. Often referred to as the “bad cholesterol,” LDL can drive the progression of atherosclerosis if it becomes oxidized within the walls of arteries. LDL particles exist in different sizes, ranging from large, fluffy molecules to small, dense molecules. Some evidence suggests that LDL particles increase the risk of developing heart disease, whereas the large, fluffy type of LDL may be cardioprotective.[1]
- ^ Redon, Josep; Chaves, F. Javier; Tellez-Plaza, Maria; Monleon, Daniel; Pichler, G.; Amigo, N., et al. (2018). LDL Particle Size And Composition And Incident Cardiovascular Disease In A South-European Population: The Hortega-Liposcale Follow-up Study International Journal Of Cardiology 264, .
The three basic components of the human diet. Macronutrients are consumed in large quantities and provide necessary energy for the body. They include carbohydrates, fats, and proteins.
A class of saturated fats. Medium-chain triglycerides are composed of medium-length fatty acid chains (six to 12 carbons long) bound by a glycerol backbone. They occur naturally in coconut oil, palm oil, and butter, but they can also be synthesized in a laboratory or food processing setting. Evidence suggests that MCT therapy improves cognitive function in older adults with Alzheimer's disease.[1] Examples of MCTs include caprylic acid (C8), capric acid (C10), and lauric acid (C12).
- ^ Juby, Angela G.; Blackburn, Toni E.; Mager, Diana R. (2022). Use Of Medium Chain Triglyceride (MCT) Oil In Subjects With Alzheimer's Disease: A Randomized, Double‐Blind, Placebo‐Controlled, Crossover Study, With An Open‐Label Extension Alzheimer's & Dementia: Translational Research & Clinical Interventions 8, 1.
The collection of genomes of the microorganisms in a given niche. The human microbiome plays key roles in development, immunity, and nutrition. Microbiome dysfunction is associated with the pathology of several conditions, including obesity, depression, and autoimmune disorders such as type 1 diabetes, rheumatoid arthritis, muscular dystrophy, multiple sclerosis, and fibromyalgia.
A tropical fruit of the gourd family. Monk fruit, also known as luo han guo, is used as a non-calorie sweetener. It produces a sweetness sensation that is 25-fold greater than that of sucrose.[1] Evidence from animal studies suggests that monk fruit reduces blood glucose levels by promoting insulin secretion.[2]
- ^ Itkin, Maxim; Davidovich-Rikanati, Rachel; Cohen, Shahar; Portnoy, Vitaly; Doron-Faigenboim, Adi; Oren, Elad, et al. (2016). The Biosynthetic Pathway Of The Nonsugar, High-Intensity Sweetener Mogroside V From Siraitia Grosvenorii Proceedings Of The National Academy Of Sciences 113, 47.
- ^ Suzuki, Yasushi A.; Tomoda, Mayuko; Murata, Yuji; Inui, Hiroshi; Sugiura, Masaki; Nakano, Yoshihisa (2007). Antidiabetic Effect Of Long-Term Supplementation With Siraitia Grosvenori On The Spontaneously Diabetic Goto–Kakizaki Rat The British Journal Of Nutrition 97, 4.
A type of dietary fat. Monounsaturated fats – often referred to as "healthy" fats – contain only one double bond in their chemical structure. They are typically liquid at room temperature but solidify upon chilling. Monounsaturated fats are found in nuts, avocados, and oils, such as those from olives, peanuts, or canola.
A bioactive compound found primarily in grapefruit. Naringin is a type of flavonoid. It exerts a wide range of physiological effects, including antioxidant, anti-cancer, anti-clotting, and anti-diabetic properties.[1] Gut bacteria convert naringin to naringenin. Evidence suggests that naringenin supplementation promotes thermogenesis in white adipose tissue and prevents post-dieting weight gain.[2] [3]
- ^ Jadeja, Ravirajsinh N.; Devkar, Ranjitsinh V. (2014). Polyphenols And Flavonoids In Controlling Non-Alcoholic Steatohepatitis Polyphenols In Human Health And Disease , .
- ^ Johnson, William; Stephens, Jacqueline; Greenway, Frank; Rebello, Candida J.; Lau, Frank H.; Lin, Yuan, et al. (2018). Naringenin Promotes Thermogenic Gene Expression In Human White Adipose Tissue Obesity 27, 1.
- ^ Thaiss, Christoph A; Shapiro, Hagit; Elinav, Eran (2017). Post-dieting Weight Gain: The Role Of Persistent Microbiome Changes Future Microbiology 12, 7.
A dietary pattern that promotes excessive weight gain. Obesogenic diets are typically rich in saturated fats and added sugars. Obesogenic diets may change the gut microbiome prior to the development of obesity, altering microbial metabolite production and promoting a wide range of disease states.[1]
- ^ Cacabelos, Ramón (2019). Pathoepigenetics: The Role Of Epigenetic Biomarkers In Disease Pathogenesis Pharmacoepigenetics , .
Dietary fats acids that have more than one unsaturated carbon bond in the molecule, such as omega-3 and omega-6 fatty acids. PUFAs are present in fish, nuts, and seeds and are more prone to oxidation than other fatty acids. PUFAs activate a master gene called PPAR, which is involved in lipid metabolism.
An umbrella term for the metabolic byproducts of microbial digestion of foodstuffs in the human gut. Postbiotics may trigger activation of the immune system and subsequent anti-inflammatory, antioxidant, antiviral, and cardioprotective responses.[1] Examples of postbiotics include short-chain fatty acids, microbial cell fractions, functional proteins, sugars, and others.
- ^ Knol, Jan; Roeselers, Guus; Wegh,; Geerlings,; Belzer, (2019). Postbiotics And Their Potential Applications In Early Life Nutrition And Beyond International Journal Of Molecular Sciences 20, 19.
Relating to the period after eating. Postprandial biomarkers are indicators of metabolic function. For example, postprandial hyperglycemia is an early sign of abnormal glucose homeostasis associated with type 2 diabetes and is markedly high in people with poorly controlled diabetes.
Dietary components (primarily indigestible fibers) that promote the growth and survival of beneficial microbes in the human gut. Prebiotic foods include asparagus, beets, garlic, chicory, onion, Jerusalem artichoke, grains, and breast milk.
Live bacteria in foods or supplements that, when consumed, promote or maintain a healthy population of gut microbes. Probiotic foods include yogurt, kefir, sauerkraut, and kombucha
A short-chain fatty acid produced by microbes in the gut. Propionate participates in a wide range of physiological functions, including fat metabolism, hunger control, and cancer prevention, among others.[1]
- ^ Hosseini, Elham; Grootaert, Charlotte; Verstraete, Willy; Van De Wiele, Tom (2011). Propionate As A Health-Promoting Microbial Metabolite In The Human Gut Nutrition Reviews 69, 5.
Weight gain that occurs after significant weight loss. Following weight-loss interventions, approximately one-third of lost weight is regained within one year, and half of all people who lose weight will return to their baseline weight within five years.[1] Evidence suggests that recurrent weight gain is due to pre-obesity changes in the gut microbiome that persist even after weight loss.[2]
- ^ Waldman, Scott A; Blomain, Erik Scott; Dirhan, Dara Anne; Valentino, Michael Anthony; Kim, Gilbert Won (2013). Mechanisms Of Weight Regain Following Weight Loss ISRN Obesity 2013, .
- ^ Thaiss, Christoph A; Shapiro, Hagit; Elinav, Eran (2017). Post-dieting Weight Gain: The Role Of Persistent Microbiome Changes Future Microbiology 12, 7.
An artificial sweetener that is approximately 300 to 400 times sweeter than sucrose but carries a slight bitter or metallic aftertaste. Saccharin was banned in 1975 in the United States due to its purported cancer-promoting properties. The ban was lifted in 1991, but saccharin and foods and drinks containing saccharin were required to carry a warning label. In 2000, legislation reversed the label requirement.
A type of dietary fat. Saturated fats – often referred to as "unhealthy" fats – contain no double bonds in their chemical structure. They are typically solid at room temperature and are found in butter, palm and coconut oils, cheese, and red meat. Robust scientific evidence suggests that saturated fats contribute to cardiovascular disease, especially in the setting of a diet high in refined sugars.[1] [2]
- ^ Krauss, Ronald M; Kris-Etherton, Penny M (2020). Public Health Guidelines Should Recommend Reducing Saturated Fat Consumption As Much As Possible: YES The American Journal Of Clinical Nutrition 112, 1.
- ^ Chunchai, Titikorn; Apaijai, Nattayaporn; Arinno, Apiwan; Palee, Siripong; Pratchayasakul, Wasana; Kerdphoo, Sasiwan, et al. (2018). High‐Saturated Fat High‐Sugar Diet Accelerates Left‐Ventricular Dysfunction Faster Than High‐Saturated Fat Diet Alone Via Increasing Oxidative Stress And Apoptosis In Obese‐Insulin Resistant Rats Molecular Nutrition & Food Research 63, 2.
Fatty acids that contain fewer than six carbons in their chemical structure. SCFAs are produced by the gut microbiota during the fermentation of dietary fiber. They provide energy to colonic cells and are crucial to gut health. In addition, SCFAs may play roles in the prevention and treatment of metabolic syndrome, inflammatory bowel disorders, and certain types of cancer. Some evidence suggests SCFAs can cross the blood-brain barrier to affect brain function. The principal SCFAs produced in the human gut are acetate, propionate, and butyrate.
A non-nutritive sweetener derived from the leaves of the stevia plant. Stevia tastes approximately 300 times sweeter than sucrose. Evidence suggests that stevia exerts antioxidant, antimicrobial, antifungal, and anticarcinogenic properties in humans.[1]
A tiny region located in the hypothalamus responsible for controlling circadian rhythms. The SCN maintains control across the body by synchronizing "slave oscillators," which exhibit their own near-24-hour rhythms and control circadian phenomena in local tissue.
Trimethylamine (TMA) is an ammonia-like compound produced by certain gut microbes during the metabolism of L-carnitine. It is taken up in the gut and converted to trimethylamine N-oxide (TMAO). Evidence suggests that TMAO promotes atherosclerosis, driving the pathogenesis of cardiovascular disease. The mechanisms that drive this association may be related to foam cell formation and impaired reverse cholesterol transport from atherosclerotic plaques.[1]
- ^ Smith, J D; Tang, W.H. Wilson; Bushman, Frederic; Levison, Bruce; Org, Elin; Sheehy, Brendan T, et al. (2013). Intestinal Microbiota Metabolism Of L-Carnitine, A Nutrient In Red Meat, Promotes Atherosclerosis Nature Medicine 19, 5.
A metabolic disorder characterized by high blood sugar and insulin resistance. Type 2 diabetes is a progressive condition and is typically associated with overweight and low physical activity. Common symptoms include increased thirst, frequent urination, unexplained weight loss, increased hunger, fatigue, and impaired healing. Long-term complications from poorly controlled type 2 diabetes include heart disease, stroke, diabetic retinopathy (and subsequent blindness), kidney failure, and diminished peripheral blood flow which may lead to amputations.
An over-the-counter probiotic therapy. VSL#3 contains eight strains of lactic acid-producing bacteria (Lactobacillus plantarum, Lactobacillus delbrueckii subsp. Bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, Bifidobacterium breve, Bifidobacterium longum, Bifidobacterium infantis, Streptococcus salivarius subsp. thermophilus). In lab studies, VSL#3 has been shown to prevent the development of or ameliorate the symptoms of colitis in mice.[1]
- ^ Appleyard, Caroline B; Arthur, Janelle C; Uronis, Joshua M.; Keku, Temitope; Fodor, Anthony; Carroll, Ian M., et al. (2011). Gut Microbial Diversity Is Reduced By The Probiotic VSL#3 And Correlates With Decreased TNBS-induced Colitis Inflammatory Bowel Diseases 17, 1.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
Microbiome News
- Berberine treatment after colon polyp removal showed lasting protection against recurrence.
- Nitrate-rich beetroot juice lowered blood pressure in older adults by altering oral microbiome composition.
- Single course oral fecal microbiota transplant in adolescents with obesity linked to lower body fat and metabolic risk four years later.
- Exposure to UV radiation triggers a 30% shift in skin bacteria composition, weakening the usual immune tolerance to UV exposure.
- Gut microbiome may influence how the body handles 'forever chemicals,' with certain bacteria showing a significant ability to accumulate PFAS, potentially impacting the rate of chemical clearance from the body.