#79 Axel Montagne, Ph.D. on Blood-Brain Barrier Dysfunction in Alzheimer’s Disease and Dementia
This episode is available in a convenient podcast format.
These episodes make great companion listening for a long drive.
The Omega-3 Supplementation Guide
A blueprint for choosing the right fish oil supplement — filled with specific recommendations, guidelines for interpreting testing data, and dosage protocols.
Dr. Axel Montagne is a chancellor's fellow and group leader at the UK Dementia Research Institute at the University of Edinburgh Centre for Clinical Brain Sciences. His group aims to understand how, when, and where critical components of the blood-brain barrier become dysfunctional preceding dementia and in the earliest stages of age-related cognitive decline. With this knowledge, they hope to develop precise treatments targeting brain vasculature to protect brain function.
More importantly, his work and that of his colleagues provide a critical lens through which to view the contributions of vascular dysfunction (or, conversely, vascular health – if we choose to preserve it) as a critical common thread in dementia and neurodegeneration.
In this episode, Dr. Montagne and I discuss:
-
What dementias have in common
-
The importance of preserving small blood vessels (in the brain)
-
Changes in the blood-brain barrier in aging that cause "leaking"
-
Predicting cognitive decline early with biomarkers – an opportunity for intervention?
-
Why targeting amyloid isn’t enough
-
The impact of the APOE4 genotype on brain vasculature
-
The cause of white matter damage in the brain
-
Why the loss of omega-3 transport affects pericytes
-
The role of exercise in prevention of blood-brain barrier dysfunction
-
Why high heart rates during exercise preserve brain function
-
The role of exercise in preserving vision health
-
Why leaky vessels damage myelin and the brain
-
Can you have more than one type of dementia?
-
Does the breakdown of the blood-brain barrier cause “type 3 diabetes"?
-
Why omega-3 may prevent detachment of pericytes
-
Why a hepatitis drug restored cognition in APOE4 mice
-
Why blood-brain barrier disruption results in the accumulation of amyloid-beta
-
Why lifetime hypertension increases dementia risk
-
Effects of obesity on blood-brain barrier leakage
(For more timestamps, please click the timeline tab.)
Identifying a common threat in cognitive decline
"Many studies back from 20-30 years ago show that when you look at postmortem brain tissue samples of people that died from Alzheimer's disease, you can see a lot of vascular problems in every single case." - Axel Montagne, Ph.D. Click To Tweet

Biomarkers of blood-brain barrier breakdown and vascular injury
Rather than thinking about dementia as the amyloid-beta plaques and tau tangles characteristic of Alzheimer's disease or altered brain glucose metabolism (colloquially referred to as "type 3 diabetes"), what if we could instead find an underlying and unifying pathology involved in most dementias?
Alzheimer's disease, cerebral small vessel disease, and vascular dementia are the three most prevalent forms of dementia and can present simultaneously, exacerbating the associated cognitive losses. Although some treatments for dementia are available, they are limited and only moderately effective at slowing the insidious decline accompanying the condition – and carry many serious side effects. A fresh look at identifying and addressing the root causes of dementia has therefore taken on greater importance. The health of the blood vessels that comprise the blood-brain barrier may be one of the most critical and early pathological features of Alzheimer's disease and other dementias.
More than half of all dementias begin with the breakdown of the blood-brain barrier
Scientists now know that roughly half of all dementias start with the breakdown of the smallest vessels in the brain and subsequent leakiness of the blood-brain barrier.[1] Biomarkers of blood-brain barrier dysfunction can be measured before other pathological hallmarks of Alzheimer's disease, including amyloid plaques and tau tangles. Roughly three decades ago, researchers observed an interesting phenomenon in the post-mortem brain tissue of some people with Alzheimer's disease: a propensity for vascular dysfunction – a broad term that describes the loss of normal endothelial tone and permeability.
These losses promote the deterioration and disease of small blood vessels in the brain, ultimately compromising the blood-brain barrier, the brain's primary defense to keep the brain clear of pathogens, toxins, immune factors, and other blood components found in circulation. Breakdown of the blood-brain barrier also disrupts blood flow to the brain, decreasing the brain's supply of oxygen and glucose.
Blood-brain barrier leakage affects the brain region involved in learning and memory
The learning and memory region of the brain, the hippocampus, is leakier than normal in people that are older versus young, and we know that this region leaks the earliest in dementia as well. Click To Tweet
The loss of blood-brain barrier integrity is a feature of normal aging and typically begins in the hippocampus, the part of the brain that is crucial for memory (in particular, the consolidation of short-term memories to long-term memories), learning, and spatial navigation.[2] Consequently, the hippocampus is the first brain region to exhibit blood-brain barrier breakdown in dementia. This "leakiness" is observable in brain imaging studies, which can detect differences between young and old people's vessels – even if the latter are cognitively healthy.
In the research setting, the presence of blood-brain barrier leakiness, along with various blood and cerebrospinal biomarkers, can predict future cognitive decline. Interestingly, leaky vessels aren't always co-located with amyloid-beta and tau protein accumulation, suggesting that these pathological events may be independent pathways for the development of dementia – and that a "cocktail approach" of simultaneously addressing a leaky barrier and amyloid-beta might be productive.
The major genetic risk factor for Alzheimer's disease, APOE4, promotes earlier breakdown of the blood-brain barrier
"If you target the vessels, it can have a big impact on neuronal function and cognition."- Axel Montagne, Ph.D. Click To Tweet

APOE4 isoform-specific CypA-MMP9 activation
A variant of the APOE gene called APOE4 is the primary genetic risk factor for late-onset Alzheimer's disease. Carrying one copy of APOE4 increases a person's Alzheimer's disease risk two- to threefold; carrying two copies increases a person's risk as much as 15-fold. People with two copies of APOE4 have 2.7 times as many amyloid oligomers in their brains as people with two APOE3 alleles.
Biomarkers of and contributors to the process of blood-brain barrier breakdown, MMP9 and cyclophilin A, are elevated in the cerebrospinal fluid of people carrying the APOE4 allele. Evidence suggests APOE4 competes with amyloid-beta for binding to a receptor called LRP1, which typically participates in amyloid clearance. Binding to LRP1 results in a release of proteins, including MMP9 and cyclophilin A, that degrade the tight junctions that hold endothelial cells that comprise the blood-brain barrier, thus leading to breakdown and increased permeability.
High Levels of cyclophilin A are implicated in many diseases, including neurodegenerative and cardiovascular diseases.[3] This should give us pause because APOE4 carriers have nearly four times more MMP9 and cyclophilin A than non-carriers. However, the good news is that studies in mice that carry APOE4 have shown that inhibiting the action of cyclophilin A partially restores vascular function and cognition.
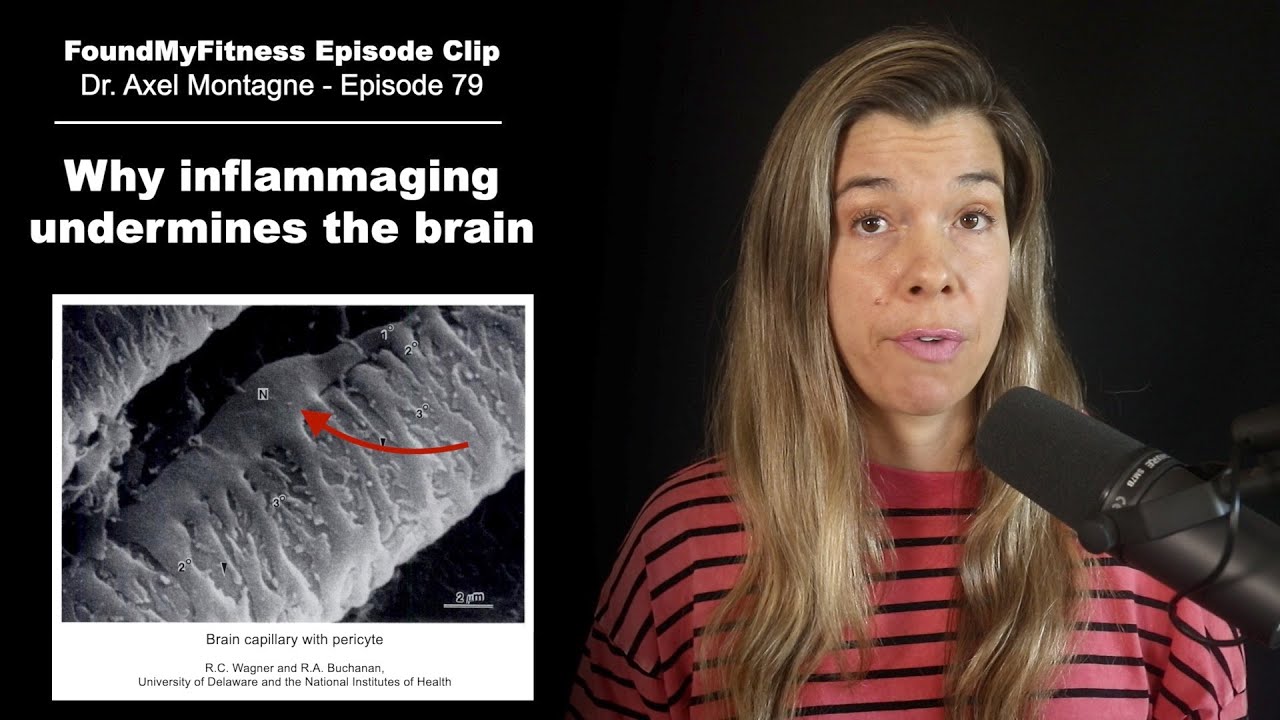
Degeneration of brain capillary pericytes leads to neurodegeneration and leaks in the blood-brain barrier
"If the pericytes are not there; you can easily assume that your blood flow will be disturbed. They are there to constrict and dilate vessels, so when they detach, not only do you have the leakage of the barrier, but you also have some blood flow problems."- Axel Montagne, Ph.D. Click To Tweet
Integral to the pathophysiology of cerebral small vessel disease and neurodegenerative diseases involving the loss of blood-brain barrier integrity is the detachment and loss of pericytes. These specialized endothelial cells cover as much as 80 percent of the surface area of brain capillaries in the cortex and hippocampus of the human brain and wrap around the smallest vessels that make up the blood-brain barrier.
Pericytes have several critical functions:
- Regulating vascular blood flow via vasoconstriction and dilation.
- Providing structural support to small blood vessels.
- Participating in regulating transcytosis in endothelial cells as part of the neurovascular unit, which makes up the blood-brain barrier.
Aging contributes to neuroinflammation, causing endothelial cells to adopt a pro-inflammatory phenotype, which is thought to ultimately drive the detachment of pericytes and contribute to the breakdown of the blood-brain barrier and the pathophysiology of various dementias, including Alzheimer's disease. In fact, it is the detachment of pericytes that enables the physical entry of immune cells_ into the brain. As pericytes detach, they shed a cell-surface protein called platelet-derived growth factor receptor-beta, or PDGFR-beta. Soluble PDGFR-beta is detectable in the cerebrospinal fluid and plasma of cognitively normal older adults and is more pronounced in people with early Alzheimer's disease, suggesting it may be an early biomarker of the disease.[4]
Preventing toxic proteins originating in blood from entering the brain
As the blood-brain barrier breaks down, whether as a part of aging or neurodegenerative disease, immune cells, inflammatory cytokines, toxic proteins, and red blood cells gain access to the brain from the blood and contribute to cognitive dysfunction.
Examples include:
- Plasminogen - A clot-busting protease that is associated with neurotoxicity, calcium-induced excitotoxicity, microglia activation, and neuronal laminin degradation, leading to neuroinflammation.
- (Pro-)thrombin - A neurotoxic clotting protein that activates microglia by upregulating pro-inflammatory pathways, such as NF-kB, and promoting further blood-brain barrier degradation and neuronal apoptosis.[5]
- Auto-antibodies - Immune cells that target neuronal receptors and synaptic proteins, driving neuroinflammation.[6]
- Albumin - A protein derived from the blood that, in the setting of a leaky blood-brain barrier, enters the brain where it can drive inflammation and mediate excitotoxicity.[7]
- Fibrinogen - A protein that plays a role in blood clotting; see section below.
Fibrinogen, a protein found in the blood, is toxic to the brain.
"It shouldn't be in the brain at all, but we start seeing this extravascular deposition of fibrinogen, which means that, for it to cross, you must have some degree of breakdown of the blood-brain barrier." - Axel Montagne, Ph.D. Click To Tweet
One example of neurotoxicity originating from plasma proteins is fibrinogen, a protein produced in the liver that plays roles in blood clot formation and is crucial for survival. But fibrinogen should not be in the brain. Disruption of the blood-brain barrier allows fibrinogen to leak into the brain. Its deposition is toxic to neurons and oligodendrocytes (myelin-producing cells) and leads to inflammation and white matter disease, a common feature of Alzheimer's disease.
Fibrinogen also activates the microglia, the brain’s resident immune cells. When inflammatory signals activate microglial cells, they switch from protecting the blood-brain barrier to attacking it. This promotes a vicious cycle of blood-brain barrier dysfunction and neurotoxicity. Chronic activation of microglia causes neuroinflammation and increases dementia risk.
Strategies of prevention for dementia
"We need to exercise no matter what. If you want to stay healthy in terms of brain function, yeah, no other choice."- Axel Montagne, Ph.D. Click To Tweet
While we await potential breakthroughs in targeting elements of the neurovascular unit in the blood-brain barrier directly with stem cell therapeutics, gene therapies, and other cutting-edge techniques, there are things we can do now to better support vascular health and thereby help maintain the integrity of the blood-brain barrier.
Omega-3 fatty acids
"As we age and with dementia, Mfsd2a, the receptor for omega-3 is reduced on the blood vessels. And where there is a reduction of Mfsd2a on blood vessels, we see pericyte loss."- Axel Montagne, Ph.D. Click To Tweet

MFSD2A is essential for blood-brain barrier integrity and DHA transport
Omega-3s, especially the marine-derived eicosapentaenoic acid, EPA, and docosahexaenoic acid, DHA, exert potent anti-inflammatory effects on the body's cells via the action of metabolic byproducts called specialized pro-resolving mediators, or SPMs. Specific classes of SPMs, such as resolvins, protectins, and maresins, have been independently shown to promote blood-brain barrier repair and downregulate neuroinflammation in the context of various neurological diseases, including dementia. These SPMs help lower inflammation, including that induced by the presence of fibrinogen.[8] [9]
The omega-3 fatty acid DHA may play a direct role in maintaining blood-brain barrier integrity. Mfsd2a, a transmembrane protein found exclusively on the endothelial cells that line blood vessels on the blood-brain barrier, is the sole means by which lysophospholipid DHA is delivered to the brain. In animal studies where Mfsd2a is defective or absent (which can occur with age or genetic predisposition), this reduces the brain's DHA levels by more than fifty percent and causes the blood-brain barrier to break down. This suggests that DHA plays a vital role in maintaining blood-brain barrier integrity.
Aerobic exercise
"If you don't make sure that your heart is pumping at a high rate regularly during the week, those tiny vessels that are even smaller than your hair in terms of diameter will start to collapse." - Axel Montagne, Ph.D. Click To Tweet
Aerobic exercise stands out as a potent tool against age-related vascular dysfunction. Exercise improves endothelial function and increases the density and overall function of brain capillaries, which comprise roughly 90 percent of the brain's vasculature. Exercise intensity may be necessary for this effect. Vigorous exercise increases vascular endothelial growth factor, resulting in increased capillary density and the sprouting of new capillaries from existing blood vessels at the blood-brain barrier.
Exercise also increases brain-derived neurotrophic factor (BDNF) at the endothelial cells that comprise the blood-brain barrier, strengthening them. The shear force of blood flow during vigorous exercise is a signal that boosts BDNF production in the brain, suggesting vigorous exercise, in particular, may be a powerful tool to prevent neurodegenerative disease.[10]
A good rule of thumb for determining your exercise intensity: It should be challenging to maintain a conversation, make you sweat, and, ideally, elevate your heart rate to 80 percent of your estimated maximum. When factors like these align, you're achieving a vigorous intensity in your exercise.
Blood pressure
"When people have hypertension, they tend to have more microbleeding in the basal ganglia. A few studies show that hypertension also triggers blood-brain barrier leakage and pericyte loss."- Axel Montagne, Ph.D. Click To Tweet
Nearly half of all adults in the United States have high blood pressure, defined as having a systolic pressure of 130 mmHg or higher or a diastolic pressure of 80 mmHg or higher. Maintaining early healthy blood pressure is critical to ensuring the proper functioning of blood vessels in the brain and blood-brain barrier integrity. Cumulative exposure to high blood pressure damages blood vessels and increases dementia risk. Aerobic exercise and sauna use have robust blood pressure-lowering effects.
Selected publications for this episode
- Interplay between Brain Pericytes and Endothelial Cells in Dementia (2021)
- Blood-brain barrier breakdown is an early biomarker of human cognitive dysfunction (2019)
- Alzheimer's disease: A matter of blood-brain barrier dysfunction? (2017)
- Blood-brain barrier breakdown in the aging human hippocampus (2015)
- APOE4 leads to blood-brain barrier dysfunction predicting cognitive decline (2020)
- Blood-Brain Barrier: From Physiology to Disease and Back (2018)
- Central nervous system pericytes in health and disease (2011)
- Transcytosis at the blood–brain barrier (2019)
Related FoundMyFitness resources
Articles:
Episodes:
- Dr. Dale Bredesen on Preventing and Reversing Alzheimer's Disease
- Intestinal Permeability: The Bacterial link to Aging, Brain Barrier Dysfunction & Metabolic Disorder
About Dr. Axel Montagne
People mentioned in this episode
- Joanna Wardlaw, MD
- Berislav Zlokovic, MD, PhD
- Tony Wyss-Coray, PhD
- Katerina Akassoglou, PhD
- William A. Banks, MD
- ^ Benzinger, Tammie; Morris, John C; Sweeney, Melanie D; Harrington, M G; Sepehrband, Farshid; Zlokovic, Berislav V, et al. (2019). Blood–brain Barrier Breakdown Is An Early Biomarker Of Human Cognitive Dysfunction Nature Medicine 25, 2.
- ^ Montagne, Axel; Sagare, Abhay P.; Toga, Arthur W.; Liu, Collin Y.; Amezcua, Lilyana; Chui, Helena C., et al. (2015). Blood-Brain Barrier Breakdown In The Aging Human Hippocampus Neuron 85, 2.
- ^ Pompilio, Giulio; Nigro, Patrizia; Capogrossi, M C (2013). Cyclophilin A: A Key Player For Human Disease Cell Death & Disease 4, 10.
- ^ Wang, Yan-Jiang; Wang, Jun; Fan, Dong-Yu; Li, Hui-Yun; He, Chen-Yang; Shen, Ying-Ying, et al. (2022). Dynamic Changes Of CSF sPDGFRβ During Ageing And AD Progression And Associations With CSF ATN Biomarkers Molecular Neurodegeneration 17, 1.
- ^ Jeon, Min-Tae; Kim, Kyu-Sung; Kim, Eun Seon; Lee, Suji; Kim, Jieun; Hoe, Hyang-Sook, et al. (2021). Emerging Pathogenic Role Of Peripheral Blood Factors Following BBB Disruption In Neurodegenerative Disease Ageing Research Reviews 68, .
- ^ Platt, Maryann; Agalliu, Dritan; Cutforth, Tyler (2017). Hello From The Other Side: How Autoantibodies Circumvent The Blood–Brain Barrier In Autoimmune Encephalitis Frontiers In Immunology 8, .
- ^ LeVine SM (2016). Albumin and multiple sclerosis. BMC Neurol 16, .
- ^ Valente, Mariarosaria; Dentoni, Marta; Bellizzi, Fabrizio; Kuris, Fedra; Gigli, Gian Luigi (2022). Specialized Pro-Resolving Mediators In Neuroinflammation: Overview Of Studies And Perspectives Of Clinical Applications Molecules 27, 15.
- ^ Francos-Quijorna I; Santos-Nogueira E; Gronert K; Sullivan AB; Kopp MA; Brommer B, et al. (2017). Maresin 1 Promotes Inflammatory Resolution, Neuroprotection, and Functional Neurological Recovery After Spinal Cord Injury. J Neurosci 37, 48.
- ^ Prigent-Tessier, Anne; Marie, Christine; Quirié, Aurore; Maguin-Gaté, Katy; Szostak, Justyna; Mossiat, Claude, et al. (2013). Physical Training And Hypertension Have Opposite Effects On Endothelial Brain-Derived Neurotrophic Factor Expression Cardiovascular Research 100, 3.
-
Alzheimer's as a vascular disease
-
How approximately three decades ago, researchers observed a phenomenon in the post-mortem brain tissue of people with Alzheimer's disease that eventually led to a new paradigm in viewing dementia.
-
How evidence reveals that endothelial cells in the brain's delicate capillaries adopt a proinflammatory phenotype and become leaky during normal aging. A process that accelerates during dementia occurring early in the disease may provide an essential window for intervention. 1
-
What dementias have in common
-
How the three most prevalent forms of dementia, Alzheimer's disease, cerebral small vessel disease, and vascular dementia, can present simultaneously. Researchers are interested in identifying and addressing the root causes of dementia and determining how vascular dysfunction plays a role.
-
The importance of preserving small blood vessels (in the brain)
-
Changes in the blood-brain barrier in aging that cause "leaking"
-
How specialized brain scans called Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) can detect and quantify areas of leakiness in the blood-brain barrier in living people's brains. 1
-
How during normal aging and dementia, loss of blood-brain barrier integrity typically begins in the hippocampus – the brain's learning and memory center. 1
-
Predicting cognitive decline early with biomarkers – an opportunity for intervention?
-
How scientists are developing new blood and cerebrospinal biomarkers that indicate blood-brain barrier leakiness and serve to predict future cognitive decline. 1
-
Why targeting amyloid isn’t enough
-
How people with early cognitive dysfunction develop brain capillary damage irrespective of other pathological hallmarks of Alzheimer's disease, including amyloid plaques and tau tangles. These findings suggest that these pathological events may be independent pathways for the development of dementia. 1
-
How there are currently two schools of thought in Alzheimer's research. One considers that amyloid buildup occurs rapidly and early in the disease process, leading to disrupted vessels, while the other believes that early vascular problems precede amyloid deposition.
-
How neuroimaging and plasma biomarkers can detect vascular dysfunction in cognitively normal people. 1
-
The impact of the APOE4 genotype on brain vasculature
-
The major genetic risk factor for Alzheimer's disease, APOE4, promotes earlier breakdown of the blood-brain barrier leading to reduced blood flow and blood-brain barrier leakage preceding cognitive decline. 1
-
How targeting vulnerable cells, such as pericytes and endothelial cells, that form the neurovascular unit using stem cell therapeutics, gene therapies, and other cutting-edge techniques is promising for maintaining blood-brain barrier integrity.
-
How screening people for the APOE4 genotype, in addition to a combination of blood and cerebrospinal fluid biomarkers of blood-barrier leakage, may better inform treatment goals.
-
The cause of white matter damage in the brain
-
Degeneration of pericytes – specialized endothelial cells in the brain – leads to neurodegeneration and leaks in the blood-brain barrier. 1
-
Pericytes, which wrap around and protect the tiny capillaries in the brain, have contractile proteins, allowing them to constrict and dilate vessels. Thus, pericyte detachment leads to reduced blood flow and blood-brain barrier leaks. 1
-
The role of inflammation in pericyte loss
-
Mounting evidence suggests that our endothelial cells become inflamed, driving pericytes to detach as we age. The detachment of pericytes enables the physical entry of immune cells into the brain. Furthermore, pericytes have a diminished ability to reattach in the aged brain. 1
-
Omega-3 and the blood-brain barrier
-
How marine omega-3 fatty acid metabolites known as specialized resolving mediators (SPMs) may participate in the resolution of inflammation. 1
-
How the omega-3 transporter gene MFSD2a is decreased at sites of blood-brain barrier leakage and pericyte loss. 1
-
In animal studies omega-3 transport has been shown to regulate blood-brain barrier function via MFSD2a transporter. 1
-
Why the loss of omega-3 transport affects pericytes
-
The role of exercise in prevention of blood-brain barrier dysfunction 1
-
Why high heart rates during exercise preserve brain function
-
The role of exercise in preserving vision health
-
Alzheimer’s disease vs. small vessel disease
-
Blood-brain barrier leakage is observable in brain imaging studies, such as magnetic resonance imaging (MRI) using specialized contrast agents.
-
Why leaky vessels damage myelin and the brain
-
Ways to detect microvessel damage in the brain
-
CADASIL and CARASIL are two heritable forms of white matter disease. These rare conditions provide a unique opportunity to study the blood-brain barrier's role in cerebral small vessel disease. 1
-
Can you have more than one type of dementia?
-
Does the breakdown of the blood-brain barrier cause “type 3 diabetes"?
-
Does omega-3 prevent age-related changes to brain energy metabolism? 1
-
Why omega-3 may prevent detachment of pericytes
-
Old blood vs. young blood: how does it affect the blood-brain barrier? 1
-
What’s in the blood that harms the brain? Can it be blocked?
-
-
What cells produce APOE and how it effects on Alzheimer’s risk
-
How APOE4 promotes destruction of the BBB through the cyclophilin A cascade. 1
-
Why a hepatitis drug restored cognition in APOE4 mice
-
Why blood-brain barrier disruption results in the accumulation of amyloid-beta
-
Glymphatic system vs. the IPAD pathway
-
Lecanemab and amyloid-targeting drugs 1
-
Why lifetime hypertension increases dementia risk
-
-
Why sauna may improve blood pressure and dementia risk. 1
-
Effects of alcohol on the blood-brain barrier
-
Effects of obesity on blood-brain barrier leakage
-
What if leakage of the brain barrier actually causes obesity? 1
-
Dr. Montagne’s biomarker timeline expectations
This transcript is reserved for members.
FoundMyFitness Members get access to exclusive content not available anywhere else, including a transcript of this episode.
You wouldn't believe how cool being a premium member of the world's best cross-disciplinary science-focused website and podcast really is.
A protein present in the human brain, found primarily at the synapses – the junctions between neighboring neurons where the exchange of electrical signals and neuronal communication occurs. Aggregation, or clumping, of alpha-synuclein proteins is a hallmark of Parkinson's disease, a neurodegenerative disorder of the central nervous system. Hsp70, a heat shock protein, has been shown to reduce formation of alpha-synuclein oligomers and reduce associated toxicity.[1]
- ^ Hashimoto, Tadafumi; J. McLean, Pamela; Danzer, Karin M.; Ruf, Wolfgang P.; Putcha, Preeti; Joyner, Daniel, et al. (2010). Heat‐shock Protein 70 Modulates Toxic Extracellular Α‐Synuclein Oligomers And Rescues Trans‐Synaptic Toxicity The FASEB Journal 25, 1.
A neurodegenerative disorder characterized by progressive memory loss, spatial disorientation, cognitive dysfunction, and behavioral changes. The pathological hallmarks of Alzheimer's disease include amyloid-beta plaques, tau tangles, and reduced brain glucose uptake. Most cases of Alzheimer's disease do not run in families and are described as "sporadic." The primary risk factor for sporadic Alzheimer's disease is aging, with prevalence roughly doubling every five years after age 65. Roughly one-third of people aged 85 and older have Alzheimer's. The major genetic risk factor for Alzheimer's is a variant in the apolipoprotein E (APOE) gene called APOE4.
A toxic 42 amino acid peptide that aggregates and forms plaques in the brain with age. Amyloid-beta is associated with Alzheimer's disease, a progressive neurodegenerative disease that can occur in middle or old age and is the most common cause of dementia. Heat shock proteins have been shown to inhibit the early aggregation of amyloid beta 42 and reduce amyloid beta plaque toxicity [1].
Hard, insoluble clumps of amyloid-beta protein. Amyloid plaques are a pathological hallmark of Alzheimer's disease. Formation of amyloid plaques occurs well before symptoms of Alzheimer's disease manifest.
One of three common genetic variants of the APOE (apolipoprotein E) gene. The APOE4 allele, which is present in approximately 10-15% of people, increases the risk of developing Alzheimer's disease and lowers the age of onset. Having one copy of E4 increases risk 2- to 3-fold, while having two copies increases risk as much as 15-fold.
A lipoprotein produced in the liver and the brain. In the brain, ApoE transports fatty acids and cholesterol to neurons. In the bloodstream, it binds and transports cholesterol, bringing it to tissues and recycling it back to the liver. Approximately 25% of people carry a genetic variant of this lipoprotein called ApoE4, which is associated with higher circulating levels of LDL cholesterol and a 2- to 3-fold increased risk of developing Alzheimer's disease.
Star-shaped cells found in the brain and spinal cord. Astrocytes facilitate neurotransmission, provide nutrients to neurons, maintain neuronal ion balance, and support the blood-brain barrier. Astrocytes also play a role in the repair and scarring process of the brain and spinal cord following traumatic injuries.
An intracellular degradation system involved in the disassembly and recycling of unnecessary or dysfunctional cellular components. Autophagy participates in cell death, a process known as autophagic dell death. Prolonged fasting is a robust initiator of autophagy and may help protect against cancer and even aging by reducing the burden of abnormal cells.
The relationship between autophagy and cancer is complex, however. Autophagy may prevent the survival of pre-malignant cells, but can also be hijacked as a malignant adaptation by cancer, providing a useful means to scavenge resources needed for further growth.
A group of structures in the brain that participate in the initiation and control of movement as well as some cognitive functions. The basal ganglia are embedded deep within the brain's hemispheres and are networked with several other brain structures, including the cerebral cortex, thalamus, and brainstem. Disruption of the basal ganglia network contributes to the pathogenesis of several movement disorders, including Parkinson's disease.
A measurable substance in an organism that is indicative of some phenomenon such as disease, infection, or environmental exposure.
A highly selective semi-permeable barrier in the brain made up of endothelial cells connected by tight junctions. The blood-brain barrier separates the circulating blood from the brain's extracellular fluid in the central nervous system. Whereas water, lipid-soluble molecules, and some gases can pass through the blood-brain barrier via passive diffusion, molecules such as glucose and amino acids that are crucial to neural function enter via selective transport. The barrier prevents the entry of lipophilic substances that may be neurotoxic via an active transport mechanism.
Clear, colorless liquid present in the brain ventricles and the cranial and spinal subarachnoid spaces. CSF provides protection for the central nervous system and plays a prominent role in brain development and neuronal functioning. CSF is renewed about four times every 24 hours. Low CSF turnover rate, which occurs with aging, leads to accumulation of catabolites in the brain and CSF – such as amyloid beta – that are often observed in certain neurodegenerative conditions such as Alzheimer's disease.
A broad category of small proteins (~5-20 kDa) that are important in cell signaling. Cytokines are short-lived proteins that are released by cells to regulate the function of other cells. Sources of cytokines include macrophages, B lymphocytes, mast cells, endothelial cells, fibroblasts, and various stromal cells. Types of cytokines include chemokines, interferons, interleukins, lymphokines, and tumor necrosis factor.
A general term referring to cognitive decline that interferes with normal daily living. Dementia commonly occurs in older age and is characterized by progressive loss of memory, executive function, and reasoning. Approximately 70 percent of all dementia cases are due to Alzheimer’s disease.
An omega-3 fatty acid found in the human brain and the meat of fatty fish. DHA plays a key role in the development of eye and nerve tissues, and is essential for normal brain function in humans. DHA may also reduce the risk of Alzheimer’s disease1 and cardiovascular disease, and may be useful in treating certain inflammatory conditions, such as rheumatoid arthritis. Dietary sources of DHA include krill oil and the meat and roe of salmon, flying fish, and pollock. [1] Patrick, Rhonda P. "Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease." The FASEB Journal (2018): fj-201801412R.
An omega-3 fatty acid found in the meat of fatty fish. EPA reduces inflammation in the body and helps counter oxidative stress in cells. It is crucial for modulating behavior and mood and has demonstrated beneficial effects in managing anxiety and depression. EPA may reduce risk of developing certain chronic diseases such as cancer or cardiovascular disease. Dietary sources of EPA include herring, salmon, eel, shrimp and sturgeon.
A type of cell that forms the endothelium, the thin layer that lines the blood and lymphatic vessels. Endothelial cells regulate blood fluidity and fibrinolysis, vascular tone, angiogenesis, monocyte/leukocyte adhesion, and platelet aggregation. They are critical players in the body's immune response and resolution of inflammation.[1]
- ^ Kadl, Alexandra; Leitinger, Norbert (2005). The Role Of Endothelial Cells In The Resolution Of Acute Inflammation Antioxidants & Redox Signaling 7, 11-12.
Endogenous female sex hormones. Estrogens include estrone, estradiol, and estriol. They promote the development and maintenance of secondary sex characteristics in females. Estrogens regulate the menstrual cycle and play key roles in fertility and reproduction. They influence other aspects of health, too, including cognitive function, bone health, and risk of developing cardiovascular disease and cancer.
A protein produced in the liver that plays roles in blood clot formation. Low fibrinogen levels or impaired fibrinogen function can lead to hemorrhage.
Flavonoid are widely distributed in plants, fulfilling many functions. Flavonoids have been shown to have a wide range of biological and pharmacological activities in animal, human, and in-vitro studies. Examples include anti-allergic, anti-inflammatory, antioxidant, antimicrobial, anti-cancer, and anti-diarrheal activities.
A system that clears the brain of metabolites and other waste. The glymphatic system comprises a vast arrangement of interstitial fluid-filled cavities surrounding the small blood vessels that serve the brain. During sleep, these perivascular structures increase in size by more than 60 percent. This allows a “flushing” operation in which waste products can be eliminated. The glymphatic system also facilitates the distribution of essential nutrients such as glucose, lipids, and amino acids, as well as other substances, such as growth factors and neuromodulators.
A small organ located within the brain's medial temporal lobe. The hippocampus is associated primarily with memory (in particular, the consolidation of short-term memories to long-term memories), learning, and spatial navigation. Amyloid-beta plaque accumulation, tau tangle formation, and subsequent atrophy in the hippocampus are early indicators of Alzheimer’s disease.
An amino acid present in the blood. Homocysteine is produced during the metabolism of methionine. Abnormalities in methionine metabolism can lead to elevated homocysteine levels, a condition called hyperhomocysteinemia. Elevated homocysteine levels can contribute to arterial plaque formation and increase the risk of clot formation. Some evidence suggests that elevated homocysteine levels double the risk of developing Alzheimer’s disease. Homocysteine levels vary according to dietary intake, with highest levels associated with consumption of animal protein. Variants in the genes that encode for the enzymes that metabolize homocysteine, specifically MTHFR, or methylenetetrahydrofolate reductase, markedly increase the risk of developing a wide array of diseases, including cardiovascular disease, Alzheimer’s disease, and cancer. High intake of dietary folate (present in leafy greens and other fruits and vegetables) can modulate the harmful effects associated with MTHFR.
A region of the forebrain below the thalamus that coordinates both the autonomic nervous system and the activity of the pituitary, controlling body temperature, thirst, hunger, and other homeostatic systems, and involved in sleep and emotional activity.
The chronic, low-grade inflammation that occurs with aging.[1] Inflammaging is often referred to as "sterile" inflammation because it involves minor immune cell infiltration in the absence of a pathogen.[2] The processes that drive inflammaging and the pathological conditions that arise because of it are bidirectional and involve multiple physiological processes and pathways.
- ^ Franceschi, Claudio; Bonafè, Massimiliano; Valensin, Silvana; Olivieri, Fabiola; De Luca, Maria; Ottaviani, Enzo, et al. (2006). Inflamm-aging: An Evolutionary Perspective On Immunosenescence Annals Of The New York Academy Of Sciences 908, 1.
- ^ Franceschi, Claudio; Garagnani, Paolo; Parini, Paolo; Santoro, Aurelia; Giuliani, Cristina (2018). Inflammaging: A New Immune–Metabolic Viewpoint For Age-Related Diseases Nature Reviews Endocrinology 14, 10.
A member of the specialized pro-resolving mediator family of polyunsaturated fatty acid metabolites. Maresin is produced in macrophages during the metabolism of docosahexaenoic acid (DHA). It exerts anti-inflammatory properties.
An insulating sheath composed of protein and fats that surrounds nerves. Myelin facilitates neural transmission by promoting rapid impulse conduction along axons. It is produced by the Schwann cells in the peripheral nervous system and by the oligodendrocytes in the central nervous system. Demyelinating diseases, such as multiple sclerosis, damage myelin, slowing nerve impulses and eliciting a wide range of neurological complications.[1]
- ^ Love S (2006). Demyelinating diseases. J Clin Pathol 59, 11.
A rapid-acting transcription factor that responds to harmful cellular stimuli, such as reactive oxygen species, IL-1B, bacterial endotoxin (lipopolysaccharide or "LPS"), ionizing radiation, and oxidized LDL. Incorrect regulation of NF-kB has been linked to cancer, inflammatory and autoimmune diseases, septic shock, viral infection, and improper immune development. Several viruses, including the AIDS virus HIV, have binding sites for NF-kB. In the case of HIV, the presence of NF-kB is believed to be involved in switching the virus from a latent to an active state.
A type of glial cell that is involved in the production of myelin, providing support and insulation to axons in the central nervous system. A single oligodendrocyte can extend its processes to 50 axons, wrapping approximately 1 micrometer of myelin sheath around each axon.
A type of polyunsaturated fat that is essential for human health. Omega-3 fatty acids influence cell membrane integrity and affect the function of membrane-bound cellular receptors. They participate in pathways involved in the biosynthesis of hormones that regulate blood clotting, contraction and relaxation of artery walls, and inflammation. They have been shown to help prevent heart disease and stroke, may help control lupus, eczema, and rheumatoid arthritis, and may play protective roles in cancer and other conditions. Omega-3 fatty acids include alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acid (DHA). ALA is found mainly in plant oils such as flaxseed, soybean, and canola oils. DHA and EPA are found in fish and other seafood. The human body can convert some ALA into EPA and then to DHA, but the efficiency of the process varies between individuals.
A laboratory procedure in which the circulatory systems of two distinct organisms are surgically joined, creating a single, shared physiological system. Parabiosis facilitates the study of normal physiology as well as pathological states, such as obesity, diabetes, and the aging process. Studies using heterochronic parabiosis (the joining of organisms of dissimilar ages) have demonstrated that the blood of a young animal has restorative effects on its conjoined, older partner, rejuvenating tissues of the nervous system, skeletal muscle, heart, liver, and other organs.[1]
- ^ Ashapkin, Vasily V.; Kutueva, Lyudmila I.; Vanyushin, Boris F. (2020). The Effects Of Parabiosis On Aging And Age-Related Diseases Advances In Experimental Medicine And Biology , .
A mixture of solid particles and liquid droplets. It is present in fine inhalable particles, with diameters that are generally 2.5 micrograms or less. Exposure to air pollution promotes oxidative stress and increases the risk of developing many chronic diseases, including cardiovascular disease, cancer, hypertension, and diabetes. Evidence indicates that global air pollution shortens people’s lives on a scale greater than warfare, other forms of violence, parasitic infection, and more.
The observable physical characteristics of an organism. Phenotype traits include height, weight, metabolic profile, and disease state. An individual’s phenotype is determined by both genetic and environmental factors.
Capable of developing into any type of cell or tissue except those that form a placenta or embryo.
A member of the specialized pro-resolving mediator family of polyunsaturated fatty acid metabolites. Resolvin is produced during the metabolism of omega-3 fatty acids, primarily eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), as well as docosapentaenoic acid (DPA) and clupanodonic acid. It exerts anti-inflammatory effects.
Byproducts of omega-3 fatty acid metabolism. SPMs reduce the inflammation that drives many chronic diseases. Four families of SPMs have been identified and include the resolvins, lipoxins, protectins, and maresins. The SPMs promote apoptosis, regulate leukocyte (white blood cell) activity, and reduce the production of proinflammatory mediators.
Abnormal aggregates of hyperphosphorylated tau, a protein found in the brain. Tau tangles are associated with traumatic brain injury and chronic traumatic encephalopathy and are one of the defining characteristics of Alzheimer’s disease. They inhibit normal brain function, and the degree of cognitive impairment in diseases such as Alzheimer’s is significantly correlated with their presence.
A progressive worsening of memory and other cognitive functions that is thought to be due to chronic reduced blood flow to the brain which is commonly due to the accumulation of cholesterol and other substances in the blood vessel walls that obstruct the flow of blood to the brain.
An excess of visceral fat, also known as central obesity or abdominal obesity. Visceral fat, in contrast to subcutaneous fat, plays a special role involved in the interrelationship between obesity and systemic inflammation through its secretion of adipokines, which are cytokines (including inflammatory cytokines) that are secreted by adipose tissue. The accumulation of visceral fat is linked to type 2 diabetes, insulin resistance, inflammatory diseases, certain types of cancer, cardiovascular disease, and other obesity-related diseases.[1]
- ^ Fontana, Luigi; Eagon, J. Christopher; Trujillo, Maria E.; Scherer, Philipp E.; Klein, Samuel (2007). Visceral Fat Adipokine Secretion Is Associated With Systemic Inflammation In Obese Humans Diabetes 56, 4.
Member only extras:
Learn more about the advantages of a premium membership by clicking below.
Supporting our work
If you enjoy the fruits of
 , you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
, you can participate in helping us to keep improving it. Creating a premium subscription does just that! Plus, we throw in occasional member perks and, more importantly, churn out the best possible content without concerning ourselves with the wishes of any dark overlords.
Dementia News
- Listening to music and playing an instrument is linked to lower dementia risk.
- Higher blood carotenoid levels are linked to slower cognitive decline in older adults carrying the APOE ε4 Alzheimer risk allele.
- Poor oral health and function are linked to a higher risk of mild cognitive impairment and dementia later in life.
- Boosting glycogen breakdown in neurons with tau-related damage improves symptoms of neurodegeneration, a potential strategy for Alzheimer's and related diseases.
- Multidomain lifestyle program involving high-intensity exercise, diet, and cognitive training provides a 14% annual improvement in cognitive function in older adults—especially those at risk of decline.